Turneroconcha magnifica (Krylova & Sahling, 2020)
|
publication ID |
https://doi.org/10.11646/zootaxa.4808.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:6CF17F6B-78D0-4BE6-8241-FB9B8C1E0B39 |
|
DOI |
https://doi.org/10.5281/zenodo.4328398 |
|
persistent identifier |
https://treatment.plazi.org/id/03B26F11-FFD3-485A-FF1D-44DB139AFDBC |
|
treatment provided by |
Plazi (2020-07-02 08:12:25, last updated 2023-10-31 18:35:49) |
|
scientific name |
Turneroconcha magnifica |
| status |
comb. nov. |
Turneroconcha magnifica comb. nov. ( Boss & Turner, 1980)
( Figs 2–10 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 )
Type material: Holotype: MCZ N 288500 About MCZ (Alvin Dive 717) . Paratypes: MCZ (Alvin Dives 727, 879, 887, 888, 892, 895, 896, 983, 984, 986, 991); USNM (Alvin Dives 727, 984) ; ANSP (Alvin Dive 984), LACM (Alvin Dive 984), NHMUK (Alvin Dive 984), MNHN (Alvin Dive 984), SIO (Alvin Dive 984).
Type locality: Galapagos Rift vent, 0˚47.0’N, 86˚08.5’W, 2495 m, Alvin Dive 717, 21 February 1977.
Material examined: see Table 1.
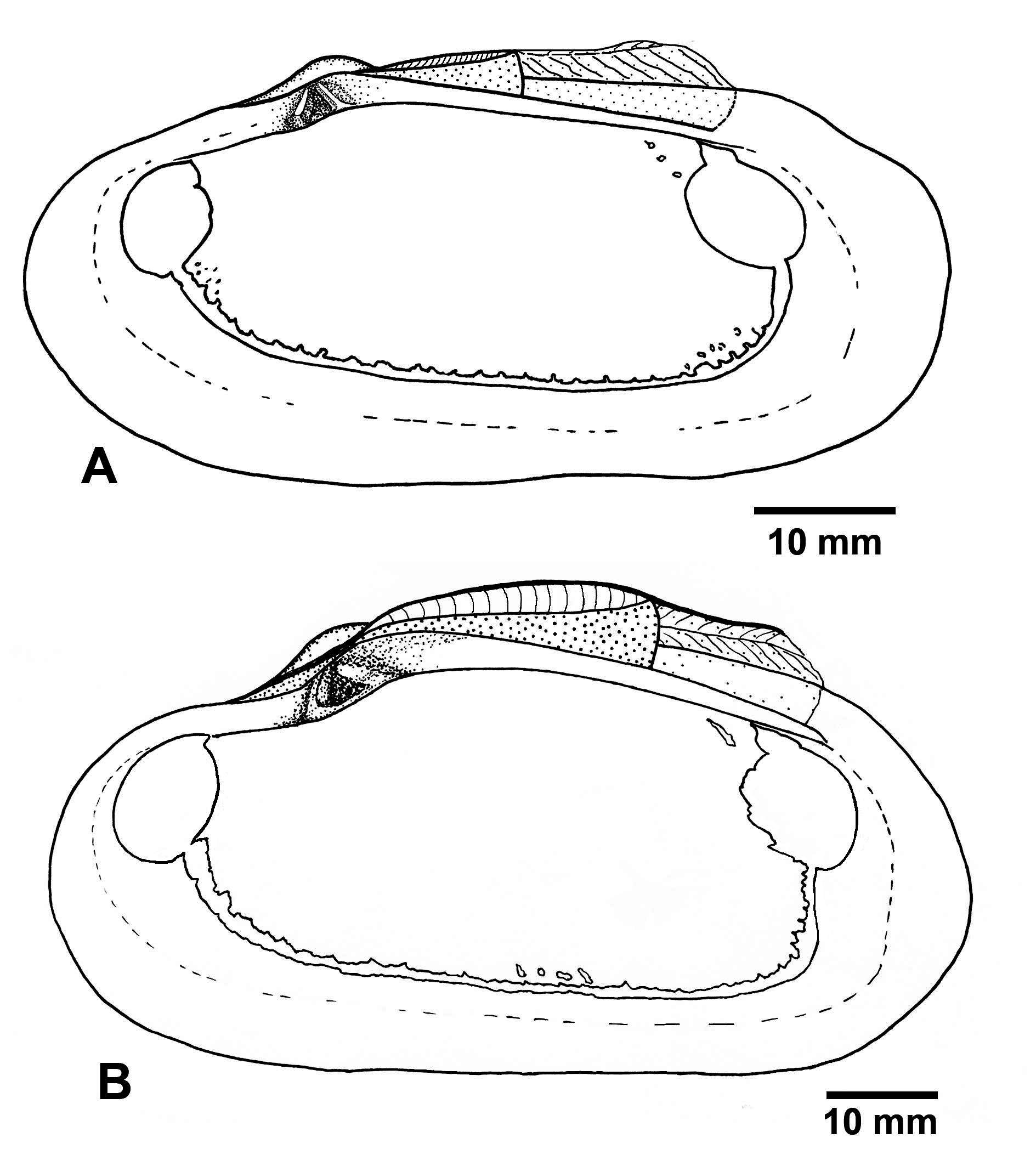
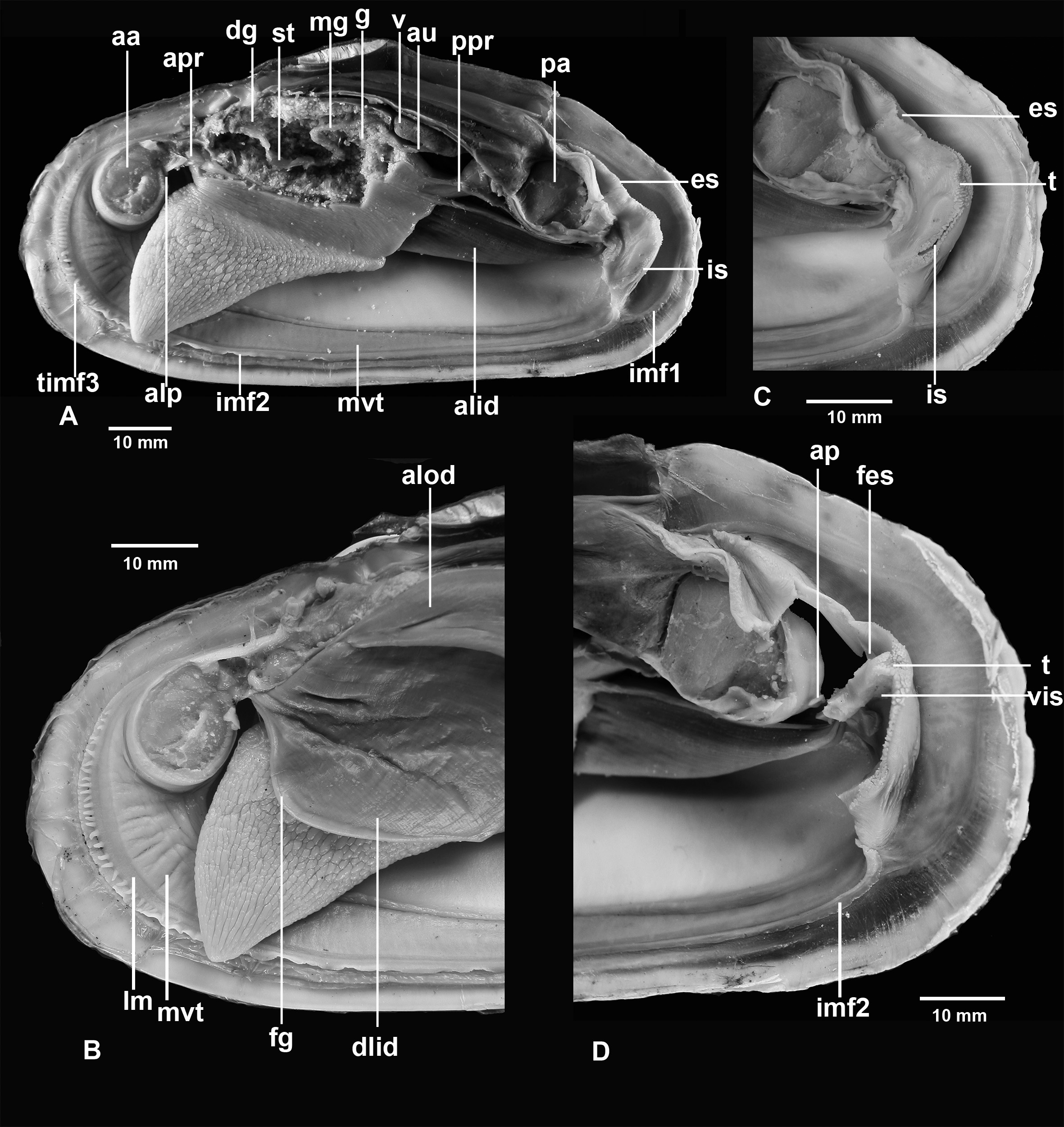
Description: Shell ( Figs 2–4 View FIGURE 2 View FIGURE 3 View FIGURE 4 ). Shell large, length to 270 mm long, thick-shelled, subovate-elongate in outline. Equivalve, inequilateral; umbos low, prosogyrate, beaks situated in about the first third of the valve ( Table 4).
Surface sculpture consists of fine irregular commarginal ridges and striae. Sometimes, a few radial thin lines run from umbo to posterior shell margin. The dorsal posterior margin can be separated by a shallow undulation. The dorsal anterior margin can be depressed just before the umbo. Periostracum thin, transparent, greyish, eroded on the umbonal part of shells in young specimens; in large specimens periostracum persistent only as a narrow zone along the shell margin, getting darker and leafy. Interior surface porcelaneous, with fine radial striation.
Pallial line starting from the postero-ventral margin of the anterior adductor scar and ending on the posteroventral margin of the posterior adductor scar. Dorsal limit of pallial line uneven because of numerous fine elongated scars extending dorsally from it. Additional small pallial attachments developed near the posterior limit of the anterior adductor scar dorsal to the pallial line. Pallial sinus absent but the pallial line often has irregular shallow posterior indentation ( Figs 2I View FIGURE 2 , 4 View FIGURE 4 ).
Anterior adductor scar rounded and shallow anteriorly and angulated and impressed posteriorly. Anterior pedal retractor scar irregularly elongate, impressed, located just above and behind the anterior adductor scar, close to it on a buttress formed by anterior thickening of the hinge margin. Posterior adductor scar slightly broader than the anterior adductor scar, rounded posteriorly, angulated anteriorly, not impressed, fused with the posterior pedal retractor scar.
Ligament strongly developed, external, parivincular. Anterior lamellar layer of ligament running from the anterior-most tooth backwards under the fibrous layer, not creating a subumbonal pit. The posterior lamellar layer running from the beak along the dorsal margin to the level of the posterior pedal retractor scar overlaying fibrous layer supported by the nymph. Posteriorly to the fibrous layer, the posterior lamellar layer attaches to a continuation of the nymph, which looks like a strong ridge deeply submerged below the dorsal shell margin.
Hinge plate relatively narrow, teeth arrangement radiating ( Figs 2 View FIGURE 2 E–G). Right valve with well-developed anterior cardinal (1) and smaller subumbonal cardinal consisting of single 3b ramus. Anterior cardinal (1) varying from wedge-shaped to shelf-like, radiating antero-ventrally. 3b thin, shelf-like, radiating postero-ventrally. Between 1 and 3b a socket for 2b. Left valve with subumbonal cardinal tooth consisting of thinner 2a ramus, radiating anteroventrally, and thicker wedge-shaped 2b ramus, radiating ventralwards. 2b often with rising higher posterior and lower anterior edges and can be fused by thin low bridge with 2a. Postero-dorsal cardinal 4b usually absent or very reduced. Hinge margin usually strongly eroded. Inner margin of valve thin, without any grooves.
Anatomy ( Figs 5–9 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 ). Mantle lobes thin, except at margins ( Figs 5–7 View FIGURE 5 View FIGURE 6 View FIGURE 7 ). Outer fold most prominent and deeply plicated. It produces a two-layered periostracum; the outer, stouter brown layer secreted within the periostracal groove between the outer fold and inner fold 1 and the inner whitish layer within pleats of the outer fold. Inner fold 1 thin, densely attached to the periostracum. Inner fold 2 thicker, fringed in the anterior third and posteriorly in front of the siphons ( Figs 6 View FIGURE 6 , 7 View FIGURE 7 ). Inner fold 3 low, better developed in the anterior part; anteriorly it bears 20-30 short tentacles, sometimes doubled, becoming longer and more numerous with age ( Fig. 7 View FIGURE 7 ).
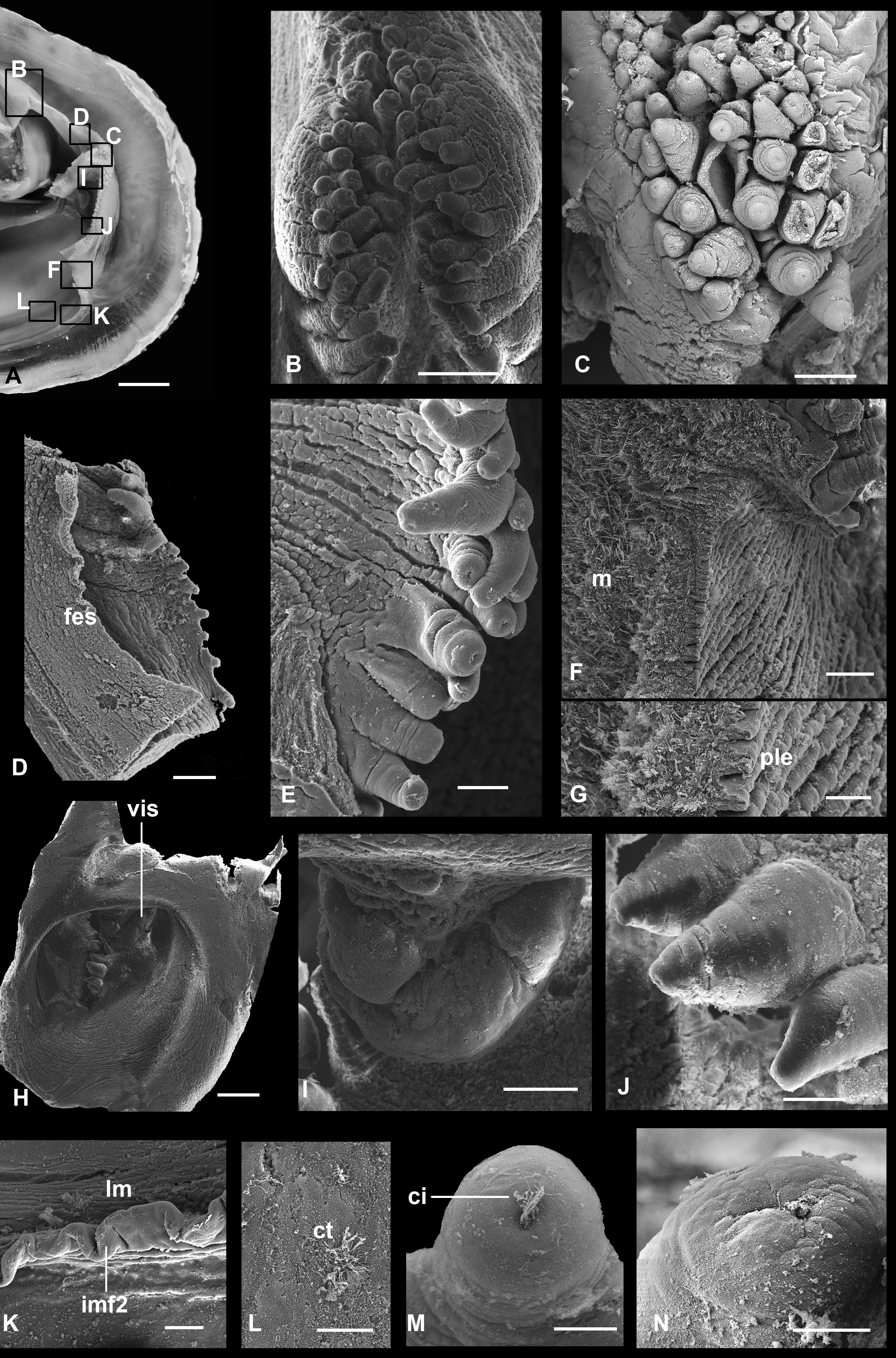
At their distal parts the tentacles are covered with ciliary tufts situated in pits ( Figs 7F, G View FIGURE 7 ). Bands of longitudinal muscles run along the base of inner fold 3. Above, there is a thickening, probably of the pallial gland, extending from the anterior to posterior adductor, parallel to the mantle margin, wider anteriorly, covered with ciliated epithelium ( Figs 7 View FIGURE 7 A–D).
Mantle wall under pallial gland vascularised; in live specimens it is possible to see that it is a sinus filled with haemolymph. Mantle fusion forms three pallial apertures, the pedal gape and two posterior siphonal openings. The pedal gape occupying the entire ventral edge from the dorsal margin of the anterior adductor to the base of the inhalant siphon. The siphons are formed from inner folds 2 and 3. Both siphons are short, the inhalant siphon more muscular and its aperture a little bit larger ( Figs 6 View FIGURE 6 , 8 View FIGURE 8 ).
The lateral margins of the inhalant siphon bear one-two rows of densely spaced stout short tentacles; lateral margins of the exhalant siphon bear one row of thin short tentacles, sometimes sparsely placed ( Fig. 8D View FIGURE 8 ), which less are conspicuous in larger specimens. Ventrally and dorsally to the siphons and between them are thickenings of the mantle margins with several rows of tentacles ( Figs 8B, C View FIGURE 8 ). All tentacles at their tips bear pits with cilia ( Figs 8M, N View FIGURE 8 ). Above the exhalant siphon, internal inner folds fuse forming a fringed margin.
Internally, the inhalant siphon on its dorsal side has a valve, a ventrally directed flap of vascularized tissue without any processes ( Fig. 8I View FIGURE 8 ). This flap can be increased in its volume and nearly entirely close the lumen of the siphon. The exhalant siphon on its internal wall has a membranous short circular flap ( Fig. 8D View FIGURE 8 ).
Foot large, very muscular, deeply rugose in its ventral part, small heel present ( Figs 5 View FIGURE 5 , 6 View FIGURE 6 ); aperture of byssal gland locates at the posterior one fourth of the sole length. Byssal groove usually short but in some clams it is nearly the length of the sole. Anterior and posterior pedal retractor muscles bifurcate before attaching to the shell.
Ctenidia non-plicate, homorhabdic, comprise two pairs of demibranchs; the length of the outer demibranch is about 85% of that of the inner and the height is half or less of that of the inner ( Fig. 5A View FIGURE 5 ). Filaments of both demibranchs reflected, constituting ascending and descending lamellae. The height of the ascending lamella of the inner demibranch is approximately 80% of that of the descending lamella; in the outer demibranch the descending lamella is about half the height of the ascending lamella. Inner demibranch with shallow food groove, which anteriorly becomes deeper and inserts at the base of the inner labial palps; outer demibranch without a food groove.
Filaments are about 45 µm in width and bear short frontal and longer latero-frontal and lateral cilia ( Figs 9J, K View FIGURE 9 ). Filaments of the same lamella connected, at approximately 150 µm intervals, with each other by tissular interfilamental junctions of about 50 µm width. These junctions contain numerous spherical cells inside ( Fig. 9H View FIGURE 9 ). Filaments together with interfilamental junctions constitute a dense net, which can be easily separated from the subfilamental part of the demibranch ( Fig. 9E View FIGURE 9 ). The subfilamental part, or bacteriocyte zone, presented by septa which extend abfrontally from filaments of both the ascending and descending lamellae and densely occupies the interlamellar space.
Every septum consists of two monolayer plates composed of bacteriocytes ( Fig. 9D View FIGURE 9 ). In the sites of interfilamental junctions pairs of adjacent plates of neighbouring filaments fuse with each other for about third of the interlamellar distance, forming tubule-like structures ( Figs 9C, H, I View FIGURE 9 ). The central area of septa is free of fusions having simple laminar structure. Margins of ctenidia fused along their entire length: anteriorly filaments inserting on the posterior surface of the anterior adductor near the inner palps; laterally dorsal margins of the ascending lamellae connected with the visceral mass; posterior to the foot, the margins of the ascending lamellae of the inner demibranch fuse with each other.
Labial palps small; outer palps consisting of low ridge with small plications, embracing anteriormost filaments of the inner demibranch; inner labial palps represented by a pair of small thickenings with plications, located between the anteriormost filaments and fusing with them along the distal margins. Inner surface of inner palps covered by cilia.
Mouth small, rounded, located posterior to the dorsal-posterior margin of the anterior adductor, opening into the oesophagus, which enters the anterior part of the stomach. Stomach thin-walled and elongated along the anteroposterior axis. Inner surface of stomach with areas of ridges and folds. The condition of the material did not allow more detailed examination. The stomach contents ( Fig. 7H View FIGURE 7 ) indicate that clams are able to filter feed. The intestine leaves at the posterior part of the stomach, running posteriorly, then it turns anteriorly and after short distance again posteriorly, passing through the visceral mass, entering the pericardial cavity, penetrating the ventricle and ending in an anus located on the posterior surface of the posterior adductor muscle ( Figs 5C View FIGURE 5 , 6A View FIGURE 6 ).
Mid-gut with major and minor typhlosoles. Before entering the pericardial cavity the intestine has a short extension, which in some specimens is separated anteriorly from the other part of the hindgut by a sphincter. In all studied specimens, of different sizes, this part of the alimentary gut was filled with detritus.
Pericardial cavity elongate, with thin transparent walls; ventricle thick-walled, elongated, large, surrounding the rectum; auricles thin-walled, with pericardial glands located on its surface, opening into the ventricle in its middle part ( Figs 5C View FIGURE 5 , 6A View FIGURE 6 ). Kidneys located between the pericardium and the posterior adductor muscle. Large gonads are embedded in the posterior-dorsal part of visceral mass, adjacent to the digestive gland and occupying the dorsal part of the foot.
Spermatozoid morphology ( Fig. 9L View FIGURE 9 ). Maximum length of head with middle piece 2.7 μm, maximum width of head 1.5 μm, nucleus barrel-shaped, acrosomal complex anterior, longitudinal profile of acrosomal vesicle low dome-shaped; 4 mitochondria.
Remarks. Boss & Turner (1980) assigned magnifica to the subgenus Ectenagena Woodring, 1938 , of the genus Calyptogena Dall, 1891 . Indeed, Turneroconcha magnifica shares some characters with Ectenagena elongata ( Dall, 1916) , the type species of Ectenagena : both of them have two pairs of demibranchs and lack the 3a-tooth and a nymphal ridge in the right valve as well as a pallial sinus. However, firstly, the hinge margin of E. elongata has a deep subumbonal pit that is absent in magnifica . Secondly, interlamellar septa in the gills of E. elongata have simple plate-like structure whereas in magnifica the interlamellar septa in the marginal area near the filaments divide into separate tubes. Thirdly, in E. elongata the posterior part of the posterior lamellar ligament is situated shallowly whilst in magnifica the posterior part of the ligament is submerged deep below the dorsal shell margin.
Turneroconcha magnifica differs from Calyptogena pacifica Dall, 1891 , the type species of the genus Calyptogena , by having two pairs of demibranchs, a Z-shaped alimentary gut, a reduced set of hinge teeth with 3a being absent, by the absence of a nymphal ridge in the right valve and a reduced 4b tooth in the left valve. In contrast, C. pacifica has a single pair of demibranchs with plate-like interlamellar septa, a linear alimentary gut, a hinge including a 3a tooth and a nymphal ridge in the right valve and a well-developed 4b tooth in the left valve ( Krylova & Sahling 2006).
Data on the morphology of spermatozoids also suggest differences between Calyptogena and T. magnifica . Within the genus Calyptogena there is some information on the morphology of spermatozoids for Calyptogena gallardoi Sellanes & Krylova, 2005 ( Parra et al. 2009) and C. pacifica ( Drozdov et al., 2019) . The head of the spermatozoid of both species of the genus Calyptogena is more elongated and bullet shaped while in T. magnifica it is barrel-shaped.
The partly tubular structure of the interlamellar septa in the gills was noted for ‘ Phreagena ’ edisonensis ( Okutani, Kojima & Kim, 2004) ( Krylova & Janssen 2006, text-fig. 6) and Christineconcha regab ( Cosel & Olu, 2009) ( Krylova & Cosel, 2011, fig. 4 B, C). A fully tubular structure of the interlamellar septa is described for species of the genus Abyssogena (Krylova et al. 2010) . From the species possessing the tubular structure of septa, T. magnifica differs by the deep location of the posterior part of the posterior lamellar ligament and a Z-shaped alimentary gut. Besides, T. magnifica lacks a pallial sinus, which is present in ‘ Ph. ’ edisonensis. T. magnifica differs from Abyssogena and Ch. regab in having two pairs of demibranchs.
The Z-shaped alimentary gut of T. magnifica is not unique among pliocardiines. Turneroconcha magnifica shares this character with Austrogena nerudai Krylova, Sellanes, Valdés & D’Elía, 2014 , the type species of the genus Austrogena Krylova, Sellanes, Valdés & D’Elía, 2014 , and the species ‘ Callocardia ’ stearnsii Dall, 1895, and ‘ Calyptogena ’ ponderosa Boss, 1968. The latter species belong to a yet undescribed genus, signified as “ cordata - group” ( Johnson et al. 2017). In contrast to T. magnifica , all these species have medium or small-sized shells with a full set of teeth and well-developed escutcheon and plate-like interlamellar septa. In Austrogena the pallial line joins the posterior adductor scar at its antero-ventral margin while in T. magnifica it ends at the postero-ventral margin of the scar. ‘ Callocardia ’ stearnsii and ‘ C. ’ ponderosa have a short pallial sinus that is absent in magnifica .
In the presence of a rather large shell lacking an escutcheon and pallial sinus, T. magnifica is similar to the fossil species Adulomya uchimuraensis Kuroda, 1931 , and Pleurophopsis unioides Van Winkle, 1919 , typifying the fossil genera Adulomya Kuroda, 1931 , and Pleurophopsis Van Winkle, 1919 , respectively. However, T. magnifica differs from them by the submergence of the posterior part of the posterior lamellar ligament and the location of the ending of the pallial line at the postero-ventral margin of the posterior adductor scar ( Table 5). In Adulomya and Pleurophopsis , the pallial line joins the posterior adductor scar at its antero-ventral margin (Krylova et al. 2010; Amano & Kiel 2011).
Amano, K. & Kiel, S. (2011) Fossil Adulomya (Vesicomyidae, Bivalvia) from Japan. The Veliger, 51, 76 - 90.
Boss, K. J. (1968) New species of Vesicomyidae from the Gulf of Darien, Caribbean Sea (Bivalvia; Mollusca). Bulletin of Marine Science, 18, 731 - 748.
Boss, K. J. & Turner, R. D. (1980) The giant white clam from the Galapagos Rift, Calyptogena magnifica species novum. Malacologia, 20, 161 - 194.
Cosel, R. von & Olu, K. (2009) Large Vesicomyidae (Mollusca: Bivalvia) from cold seeps in the Gulf of Guinea off the coasts of Gabon, Congo and northern Angola. Deep-Sea Research II, 56, 2350 - 2379. https: // doi. org / 10.1016 / j. dsr 2.2009.04.016
Dall, W. H. (1891) On some new or interesting West American shells obtained from the dredgings of the U. S. Fish Commission steamer ' Albatross' in 1888, and from other sources. Proceedings of the United States National Museum, 14, 173 - 191. https: // doi. org / 10.5479 / si. 00963801.14 - 849.173
Dall, W. H. (1895) Scientific results of explorations by the U. S. Fish Commission steamer ' Albatross'. N XXXIV. Report on Mollusca and Brachiopoda dredged in deep water, chiefly near the Hawaiian Islands, with illustrations of hitherto unfigured species from northwest America. Proceedings of the United States National Museum, 17, 675 - 733. https: // doi. org / 10.5479 / si. 00963801.17 - 1032.675
Dall, W. H. (1916) Diagnoses of new species of marine bivalve mollusks from the Northwest coast of America in the collection of the United States National Museum. Proceedings of the United States National Museum, 52, 393 - 417. https: // doi. org / 10.5479 / si. 00963801.52 - 2183.393
Drozdov, A. L., Krylova, E. M., Kudryavtsev, A. A., Galkin, S. V., Tyurin, S. A. (2019) The sperm ultrastructure and some reproductive characteristics of the chemosymbiotic bivalve Calyptogena pacifica Dall, 1891 (Vesicomyidae: Pliocardiinae). Russian Journal of Marine Biology, 45, 292 - 301. https: // doi. org / 10.1134 / S 1063074019040047
Johnson, S. B., Krylova, E. M., Audzijonyte, A., Sahling, H. & Vrijenhoek, R. C. (2017) Phylogeny and origins of chemosynthetic vesicomyid clams. Systematics and Biodiversity, 15, 346 - 360. https: // doi. org / 10.1080 / 14772000.2016.1252438
Krylova, E. M. & Sahling, H. (2006) Recent bivalve molluscs of the genus Calyptogena (Vesicomyidae). Journal of Molluscan Studies, 72, 359 - 395. https: // doi. org / 10.1093 / mollus / eyl 022
Krylova, E. M. & Janssen, R. (2006) Vesicomyidae from Edison Seamount (South Western Pacific: Papua New Guinea: New Ireland fore-arc basin) (Bivalvia: Glossoidea). Archiv fur Molluskenkunde, 135, 233 - 263. https: // doi. org / 10.1127 / arch. moll / 1869 - 0963 / 141 / 087 - 113
Krylova, E. M. & Cosel, R. von (2011) A new genus of large Vesicomyidae (Mollusca, Bivalvia, Vesicomyidae, Pliocardiinae) from the Congo margin, with the first record of the subfamily Pliocardiinae in the Bay of Biscay (northeastern Atlantic). Zoosystema, 33, 83 - 99. https: // doi. org / 10.5252 / z 2011 n 1 a 4
Krylova, E. M., Sellanes, J., Valdes, F. & D'Elia, G. (2014) Austrogena: a new genus of chemosymbiotic bivalves (Bivalvia; Vesicomyidae; Pliocardiinae) from the oxygen minimum zone off central Chile described through morphological and molecular analyses. Systematics and Biodiversity, 12 (2), 225 - 246. https: // doi. org / 10.1080 / 14772000.2014.900133
Kuroda, T. (1931) Fossil Mollusca. In: Homma, F. (Ed.), Geology of the central part of Shinano. Part 4. Kokin Shoin, Tokyo, pp. 1 - 90. [in Japanese]
Okutani, T., Kojima, S. & Kim D. (2004) A new Calyptogena Clam (Bivalvia: Vesicomyidae) from the Southwest Pacific. Venus, 63, 29 - 32.
Parra, M., Sellanes, J., Dupre, E. & Krylova, E. (2009) Reproductive characteristics of Calyptogena gallardoi Sellanes & Krylova, 2005 (Bivalvia: Vesicomyidae) from a methane seep area off Concepcion, Chile. Journal of Marine Biological Association of the United Kingdom, 89, 161 - 169. https: // doi. org / 10.1017 / s 0025315408002397
Sellanes, J., Krylova, E. (2005) A new species of Calyptogena (BIVALVIA, Vesicomyidae) from a recently discovered methane seepage area off Concepcion Bay, Chile (~ 36 ºS). 2005, Journal of Marine Biological Association of the United Kingdom, 85, 969 - 976. https: // doi. org / 10.1017 / s 0025315405011963
Van Winkle, K. (1919) Remarks on some new species from Trinidad. Bulletins of American Paleontology, 8, 19 - 27.
Woodring, W. P. (1938) Lower Pliocene mollusks and echinoids from the Los Angeles Basin, California. United States Geological Survey Professional Paper, 190, 1 - 67. https: // doi. org / 10.3133 / pp 190
FIGURE 2. Turneroconcha magnifica n. comb. (Boss & Turner, 1980), RV Western Flyer, dive D 754, L=93 mm (IORAS, BIV00036-1). A, exterior of left valve. B, interior of left valve. C, exterior of right valve. D, interior of right valve. E, left hinge plate. F, right hinge plate. G, hinge margin of both valves. H, dorsal view. I, interior of right valve, pallial scars highlighted. J, anterior view. K, ventral view. 1, ventral cardinal tooth; 2a, anterior ramus of subumbonal cardinal tooth; 2b, posterior ramus of subumbonal cardinal tooth; 3b, posterior ramus of subumbonal cardinal; 4b, reduced posterodorsal cardinal tooth; all, anterior lamellar ligament layer; F, fibrous ligament layer; ppl, posterior part of posterior lamellar ligament layer; ny, nymph, tl, trace of posterior part of anterior lamellar ligament layer.
FIGURE 3. Turneroconcha magnifica n. comb. (Boss & Turner, 1980), RV Western Flyer, dive D752, L=66 mm (IORAS, BIV00038-1) (A–D). A, interior of right valve, pallial scars highlighted. B, right hinge plate. C, interior of left valve. D, left hinge plate. RV Western Flyer, dive D754, L=36 mm (IORAS, BIV00036-2) (E, F). E, exterior of left valve. F, interior of left valve.
FIGURE 4. Turneroconcha magnifica n. comb. (Boss & Turner, 1980), semi-schematic line drawing of the interior of right valve. A, RV Western Flyer, dive D752, L=66 mm (IORAS, BIV00038-1). B, RV Western Flyer, dive D754, L=93 mm (IORAS, BIV00036-1).
FIGURE 5. Turneroconcha magnifica n. comb. (Boss & Turner, 1980), RV Western Flyer, dive D754, L=36 mm (IORAS, BIV00036-2). A, body as seen from left. B, body as seen from left, gills removed. C, body as seen from left, gills and part of visceral mass removed. aa, anterior adductor muscle; alid, ascending lamella of inner demibranch; alod, ascending lamella of outer demibranch; alp, anterior labial palps; au, auricle; dg, digestive gland; dlid, descending lamella of inner demibranch; dlod, descending lamella of outer demibranch; es, exhalant siphon; f, foot; hg, hindgut; is, inhalant siphon; k, kidney; m, mouth; mg, midgut; mvt, mantle vascularized thickening; pa, posterior adductor muscle; pc, pericardial cavity; plp, posterior labial palps; st, stomach; timf, tentacles of inner mantle fold 3; v, ventricle.
FIGURE 6. Turneroconcha magnifica n. comb. (Boss & Turner, 1980), RV Western Flyer, dive D752, L=104 mm (IORAS, BIV00038-2). A, body as seen from left, gills and part of visceral mass removed. B, anterior part of body, gills intact. C, posterior part of body. D, posterior part of body, left wall of siphons removed. aa, anterior adductor muscle; alid, ascending lamella of inner demibranch; alod, ascending lamella of outer demibranch; alp, anterior labial palps; ap, anal papilla; apr, anterior pedal retractor muscle; au, auricle; dg, digestive gland; dlid, descending lamella of inner demibranch; es, exhalant siphon; fes, inner flap of exhalant siphon; fg, food groove; g, gonad; imf1, inner mantle fold 1; imf2, inner mantle fold 2; is, inhalant siphon; lm, pallial longitudinal muscle; mg, midgut; mvt, mantle thickening covered by ciliated epithelium; pa, posterior adductor muscle; ppr, posterior pedal retractor muscle; st, stomach; t, tentacles of mantle margin between siphons; timf3, tentacles of inner mantle fold 3; v, ventricle; vis, inner valve of inhalant siphon.
FIGURE 7. Turneroconcha magnifica n. comb. (Boss & Turner, 1980), scanning electron micrographs of anatomical details, RV Western Flyer, dive D754, L=36 mm (IORAS, BIV00036-2) (A–G, I). A, mantle margin anteriorly. B, mantle margin midventrally. C, ciliated surface of mantle thickening. D, pallial longitudinal muscle. E, F, inner mantle folds 2 and 3. G, ciliary tufts of tentacles of inner fold 3. I, Cross view of mantle wall. RV Western Flyer, dive D752, L=104 mm. H, content of stomach. c, ciliated epithelium; gt, glandular tissue; hc, haemocoel; imf1, inner mantle fold 1; imf2, inner mantle fold 2; imf3, inner mantle fold 3; lm, pallial longitudinal muscle; omf, outer mantle fold; p, pits with ciliary tufts; per, periostracum; timf3, tentacles of inner mantle fold 3. Scale bars: A, B, E, 200 µm; C, D, F, 100 µm; G, 5 µm; H, 20 µm; I, 10 µm.
FIGURE 8. Turneroconcha magnifica n. comb. (Boss & Turner, 1980), scanning electron micrographs of anatomical details, RV Western Flyer, dive D 754, L=36 mm (IORAS, BIV00036-2). A, posterior part of body, black rectangles indicate locations of anatomical details shown in the following images. B, dorsal thickening of the mantle margins above exhalant siphon with three rows of tentacles. C, tentacles between siphons. D, margin of exhalant siphon from inside. E, tentacles of exhalant siphon margin. F, cross section of ventral wall of inhalant siphon. G, plicated epithelium of ventral wall of inhalant siphon. H, inhalant siphon from inside. I, inner valve of inhalant siphon from inside. J, tentacles of inhalant siphon margin. K, mantle inner fold 2 from the basis of inhalant siphon. L, ciliary tufts from inner mantle wall near siphon basis. M, tentacle of exhalant siphon. N, tentacle of inhalant siphon. ci, cilia; ct, ciliary tufts; fes, inner flap of exhalant siphon; imf2, inner mantle fold 2; lm, pallial longitudinal muscle; m, muscle bands; ple, plicated epithelium; vis, inner valve of inhalant siphon. Scale bars: A, 5 mm; B–D, I, 200 µm; E–J, K, 100 µm; G, 50 µm; H, 500 µm; L–N, 10 µm.
FIGURE 9. Turneroconcha magnifica n. comb. (Boss & Turner, 1980), Scanning electron micrographs of anatomical details, RV Western Flyer, dive D754, L=36 mm (IORAS, BIV00036-2) (A–K). A–C, transverse sections of distal parts of inner demibranch in the medial area showing interlamellar septa and lateral view of filaments. D, transverse section of filaments and interlamellar septa consisting of two plates of bacteriocytes, fusion of neighbouring plates creating dorsal wall of tubule arrowed. E, abfrontal surface of filaments. F, G, abfrontal surface of filaments with interlamellar septa. H, lateral view of filament and distal part of interlamellar septum. I, interlamellar septum showing a fragment of a tubule. J, frontal surface of filaments. K, lateral view of filament. RV Western Flyer, dive D752, L=104 mm. L, spermatozoids. acr, acrosome; fi, gill filament; fms, free margin of interlamellar septum; frc, frontal cilia; ifj, interfilamental junction; ils, interlamellar septum; lfc, latero-frontal cilia; lc, lateral cilia; mi, mitochondria; tb, boundaries between separate tubules; tub, tubule. Scale bars: A–C, E–G, 200 µm; D, H–J, 50 µm; K, 20 µm; L, 1 µm.
FIGURE 10. Turneroconcha magnifica n. comb. (Boss & Turner, 1980) in its natural habitat, Gulf of California, Alarcón Rise, 2309 m, RV Western Flyer, dive D754. A, usual epifaunal position of T. magnifica on bare basalts along crevices. B, unusual infaunal behaviour of T. magnifica in soft sediment. C, specimen with a protruding foot. D, temperature measurement in vicinity of an exhalant siphon of T. magnifica. Distance between red dots is 25 cm. Photo courtesy of MBARI with permission of R.C. Vrijenhoek.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |