Transversotrema cabrarum, Hunter, Janet A., Hall, Kathryn A. & Cribb, Thomas H., 2012
|
publication ID |
https://doi.org/ 10.5281/zenodo.214671 |
|
DOI |
https://doi.org/10.5281/zenodo.6173038 |
|
persistent identifier |
https://treatment.plazi.org/id/03AC87A1-FFB0-243F-FF27-B67814E2FE98 |
|
treatment provided by |
Plazi |
|
scientific name |
Transversotrema cabrarum |
| status |
sp. nov. |
Transversotrema cabrarum View in CoL n. sp.
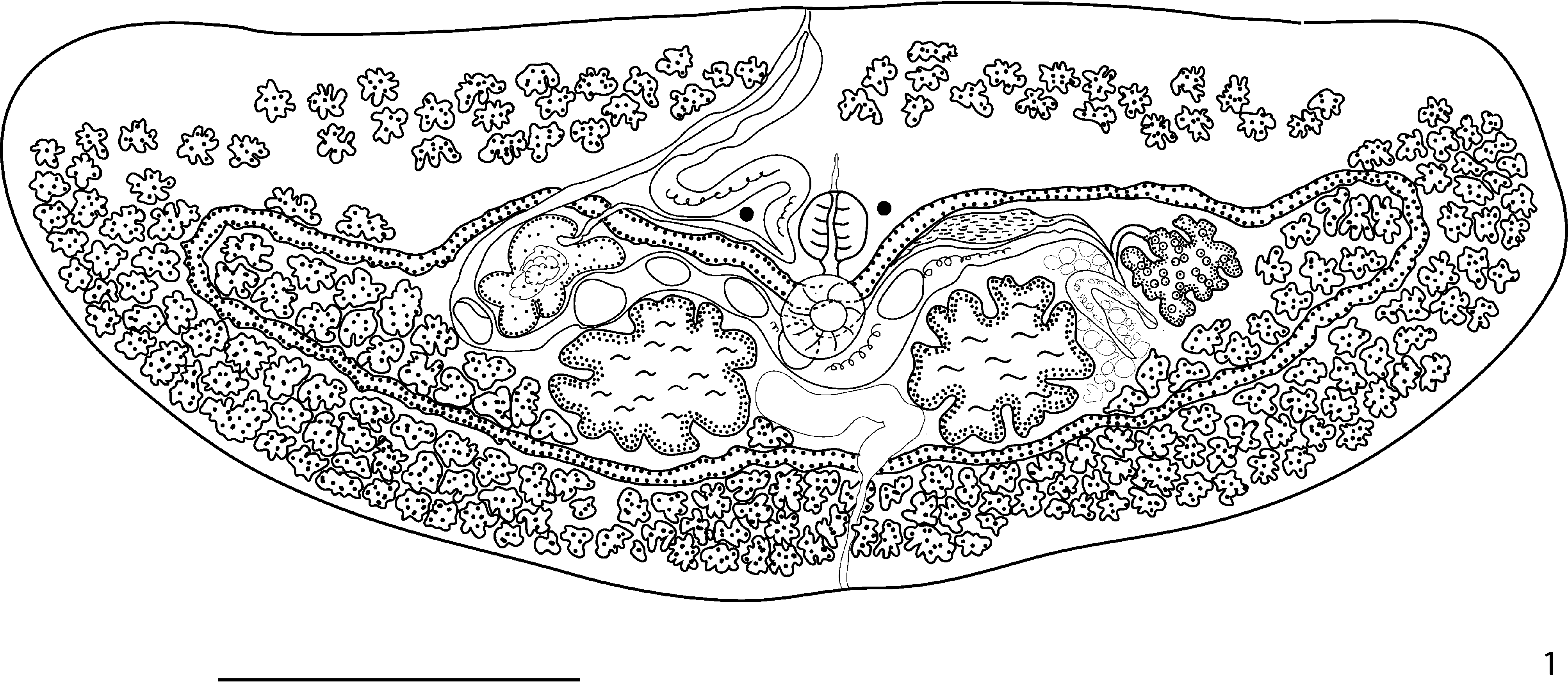
( Fig. 1 View FIGURE 1 )
Type-host: Parupeneus spilurus (Bleeker) , Mullidae , blackspot goatfish.
Site: beneath scales.
Type locality: north Tantabiddi, Ningaloo Reef, Western Australia, Australia (21°30´S 114°30´E).
Other hosts: Mullidae : Parupeneus cyclostomus (Lacepède) , goldsaddle goatfish; Parupeneus pleurostigma (Bennett) , sidespot goatfish; Parupeneus trifasciatus (Lacepède) , doublebar goatfish.
Prevalence: 8 of 15 (53%) (see Table 3)
Deposition of types: holotype (ex P . spilurus, coll. T. H. Cribb et al., 9 Aug. 2003) and 19 paratypes sent to WAM; paratypes QM G231604 (ex P . spilurus, coll. T. H. Cribb et al., 9 Aug. 2003); QM G231605–612 (ex P . spilurus, north Tantabiddi, coll. T. H. Cribb et al., 3–9 Aug. 2003); vouchers QMG231613–615 (ex P . trifasciatus, north Tantabiddi, coll. T. H. Cribb et al., 8–9 Aug. 2003), QM G231616 (ex P . cyclostomus, north Tantabiddi, coll. T. H. Cribb et al., 8 Aug. 2003), QM G231617 (ex P . pleurostigma, north Tantabiddi, coll. T. H. Cribb et al., 8 Aug. 2003), QM G231618–631 (ex P . spilurus, north Tantabiddi, coll. T. H. Cribb et al., 3–9 Aug. 2003).
Etymology: all members of this group of transversotrematids are from mullid fishes which are commonly referred to as “goatfishes”. This species name is derived from the modern Spanish cabrarum (f.), meaning “of goats”.
Description: Based on 10 (7 mature, 3 immature) specimens. Body transversely elongated, dorsoventrally flattened, widest at anterior margin, 560–910 (765) µm long, 1,498–2,512 (2,039) µm wide; total body area 0.85–2.25 (1.59) mm2; body width/length ratio 2.4–2.9 (2.7). Tegument spined; spines prominent, stout, spaced regularly in offset rows. Eyespots central in anterior half of body, 130–228 (190) µm apart; no pigment other than eyespots evident. Ventral sucker well posterior to eyespots, surface area 5,213–9,124 (7,740) µm2, with fine spines in few rings around margin. Mouth inconspicuous, mid-ventral. Pharynx prominent, large, between or slightly posterior to eyespots, 75–147 (109) µm long, 80–137 (105) µm wide. Oesophagus short 35–61 (50). Intestine present as cyclocoel, bifurcation immediately anterodorsal to ventral sucker, strongly crenulated with intestinal crenulations prominent in lateral reaches proceeds in loop laterally from central oesophagus to level of anterior margin of pharynx, then immediately passes lateroposteriorly towards each lateral margin, and then follows contour of body margin, recurving posteriorly and passing laterally to envelop gonads and some vitelline follicles. Testes paired, rounded, deeply lobed, symmetrical, not contiguous; left testis surface area 25,661–72,782 (43,204) µm2; right testis surface area 26,047–66,033 (43,842) µm2. Seminal vesicle distinctly bipartite, composed of enclosed and extracaecal portions; enclosed portion antero–dextral to right testis, saccular, distinctly lobed or entire, constricted distally to form narrow duct which passes ventral to cyclocoel and leads to extracaecal portion; extracaecal portion tubular, winding, long, passes along line of cyclocoel towards midline of body, then turns anteriorly and proceeds between eyespots, dextral to pharynx, forming naked ejaculatory duct distally. Genital pore median, on anterior margin of body. Ovary deeply lobed, sinistral to, but not contiguous with, left testis, surface area 11,400–25,514 (16,208) µm2; oviduct passes medio-posteriorly from ovary. Uterine seminal receptacle present. Laurer’s canal unites with oviduct posterior to ovary, passes and joins vitelline reservoir further posteriorly, before it opens dorsally, close to left testis; median portion of canal dilated, contains sperm and vitelline remnants. Uterus passes medially between anterior half of cyclocoel and testes, proceeds between right testis and saccular portion of seminal vesicle, then proceeds anteriorly to join ejaculatory duct at common genital pore; termination of uterus unspecialised. Vitellarium follicular; vitelline follicles scattered in extracaecal and enclosed areas of body; extracaecal follicles fill posterior region of body, extend to anterior portion of body, forming at least three rows, less dense at anterior margin of body than in posterior part of body, anterior field sometimes disrupted at lateral extremity of field; enclosed follicles in two masses, one mass in each lateral portion of enclosed area, sometimes scattered in interrupted band along inner posterior margin of cyclocoel, number 31–39 (35). Vitelline reservoir immediately anterior to left testis. Eggs tanned, no operculum observed, unembryonated in utero, 2–17 (10) per individual. Excretory bladder saccular, opens at small notch in middle of posterior margin, extends anteriorly as narrow tube and expands into transverse large sac which lies largely ventral to posterior loop of cyclocoel.
Molecular data: Sequence data from the ITS2 rDNA region were obtained for 2 specimens from P. spilurus from Ningaloo Reef (see Table 2 View TABLE 2 ). The two sequences were identical.
Remarks: Specimens of T. cabrarum n. sp. resemble T. haasi , which was described from an unknown Red Sea fish by Witenberg (1944), and also a complex of transversotrematids comprised of Transversotrema elegans Hunter, Ingram, Bray, Adlard & Cribb, 2010 , Transversotrema gigantica Hunter, Ingram, Bray, Adlard & Cribb, 2010 and Transversotrema lacerta Hunter, Ingram, Bray, Adlard & Cribb, 2010 which resemble T. haasi ; in particular, the vitelline follicles of all these species are extensive in the anterior parts of the body (Hunter et al. 2010). The current specimens from mullid fishes from Ningaloo, Australia, are conspicuously smaller than the specimens of Witenberg, rarely exceeding 2.5 mm in width, whereas the specimens of T. haasi range in width from 2.5 to 4 mm. Further, specimens of T. cabrarum n. sp. are widest at their anterior margins in contrast to T. haasi , which is widest at the equator and thus is strongly spindle-shaped (or “lancet-shaped”). Witenberg (1944) described the ejaculatory duct of T. haasi as being coiled, and in the figure (p. 179, Fig. 1 View FIGURE 1 ), the ejaculatory duct is depicted as completing one full loop immediately anterior to the pharynx; this loop was visible clearly in our examination of the type material (HUMS T-742). The ejaculatory duct of T. cabrarum n. sp. does not complete this additional loop. The oesophagus of T. haasi is relatively long and winding, in contrast to the oesophagus of T. cabrarum n. sp. which is short and straight. The overall size and shape of T. cabrarum n. sp. and the short oesophagus, in addition to the absence of a clear loop in the path of the ejaculatory duct, distinguishes it from T. haasi .
Velasquez (1975) and Cribb et al. (1992) each attributed material collected from labroid (scarid and labrid) fishes in the Western Pacific to T. haasi . Hunter et al. (2010) reported on numerous specimens from scarid and labrid fishes from Indo-Pacific waters. These were in broad agreement with those described by Velasquez (1975) and Cribb et al. (1992), but were not attributed to T. haasi , instead being recognised as a complex of three species, T. elegans , T. gigantica and T. lacerta . Molecular and morphological evidence (Hunter et al. 2010) show that T. cabrarum n. sp. from mullid fishes from Ningaloo Reef is not conspecific with T. elegans , T. gigantica or T. lacerta . Transversotrema gigantica has been reported from scarids from Heron Island and Ningaloo reef but is easily distinguished from T. cabrarum n. sp. because, although the vitelline follicles of T. cabrarum n. sp. extend into the anterior part of the body, they are scant compared to the specimens from labroid fishes, in which the vitelline follicles are dense. Transversotrema cabrarum n. sp. is also distinguished from species within the complex of species related to T. licinum ( Hunter & Cribb 2012) by the extensive distribution of vitelline follicles across the anterior margin and the short oesophagus. Finally, molecular data (see below) distinguish T. cabrarum n. sp. from all the species characterised by Hunter et al. (2010) and Hunter & Cribb (2012).
| WAM |
Western Australian Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |