Trimma finistrinum, Winterbottom, Richard, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4269.4.9 |
|
publication LSID |
lsid:zoobank.org:pub:DDEF97BF-EE79-468C-A3B1-1F8BFCB2DA17 |
|
DOI |
https://doi.org/10.5281/zenodo.6048829 |
|
persistent identifier |
https://treatment.plazi.org/id/03ABCE69-3552-A371-6DC4-FC851E59F80D |
|
treatment provided by |
Plazi |
|
scientific name |
Trimma finistrinum |
| status |
sp. nov. |
Trimma finistrinum View in CoL new species
Porthole Pygmygoby Figs. 3–5 View FIGURE 3 View FIGURE 4 View FIGURE 5 .
No published names pertain to this species.
Material examined. Holotype. ROM 101380 About ROM , 21.0 mm SL female, Fiji, between Viti Levu and Vanua Levu, back of cave at base of small vertical wall, rubble floor, 17°13'41.0"S, 178°31'58.7"E, 10.7 m, clove oil GoogleMaps , 9 Feb., 2015, J.V. Eyre.
Paratypes. BPBM 38965, 2 (23.4–25.3), Fiji, Charybdis Reef , south side (17°13.72'S, 178°2.533'E), 20 m, rotenone, 12 Mar., 2002, D.W. Greenfield, J.E. Randall, R. Langston & K. Longenecker GoogleMaps . ROM 100152, 6(10.0– 21.5), collected with the holotype.
Tissues (paratypes): T14992 (20.5); T14993 (19.2) and T14994 (10.5); collected with the holotype.
Diagnosis. A species of Trimma with bony interorbital 100% pupil diameter, fully scaled midline of nape of 12–14 scales, second dorsal spine that may reach posteriorly to middle of second dorsal fin, papillae in longitudinal row immediately below eye either single or with two papillae in vertical row (but not in vertical rows of 3–5 papillae at each position), unbranched pectoral-fin rays, usually a branched fifth pelvic-fin ray that is about half length of the fourth ray, and a large diffuse dark blotch on the posterior caudal peduncle. A colour pattern of a brownish body with most body scales having golden- to greenish-yellow (pale in preservative) centres is unique among species of Trimma .
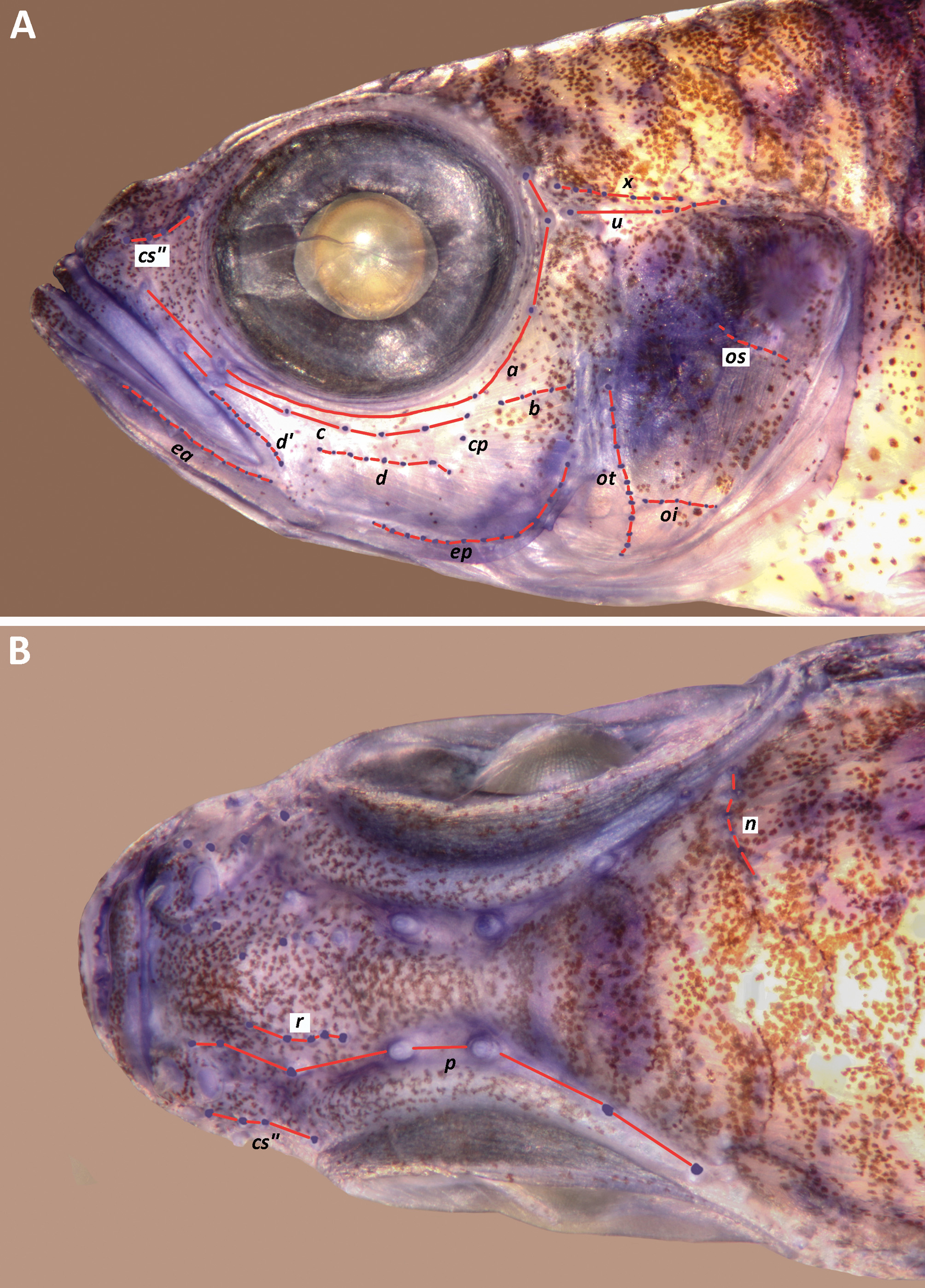
Description. The description is based on up to 12 specimens, 10.0– 25.3 mm SL (mean =16.8) from 2 lots collected off the north and east coasts of Viti Levu, Fiji. Three of these specimens were collected for analysis of the COI gene (see Discussion). Dorsal fin VI + I 8, second spine slightly to moderately elongated, reaching to between base of spine and of 6th ray of second dorsal fin, all fin rays branched except for posterior element of last ray, which reaches posteriorly 39– 43 –55% (mean = 44.7%, n = 4) distance between its base and first exposed dorsal procurrent caudal fin ray; anal fin I 8, all fin rays branched except for posterior element of last ray, which reaches posteriorly 30– 37 –49% (mean = 37.9%, n = 4) distance between its base and first exposed ventral procurrent caudal fin ray; pectoral fin 14 (n = 11), all rays unbranched, fin reaching posteriorly to above area between urogenital papilla and spine of anal fin; pelvic fin I 5, fifth ray with single dichotomous branch (n = 9) except for left side of holotype and one other specimen, where unbranched, and 47– 53 –61% (mean = 53.8, n = 10) length of fourth ray, which reaches posteriorly to between bases of anal fin rays 1 –3 (mean = 2, n = 10), pelvic rays 1–4 with a single sequential branch point; basal membrane vestigial, no pelvic fraenum. Lateral scales 23– 24 (mean = 23.1, n = 9); anterior transverse scales 8 –9 (mean = 8.2, n = 9); posterior transverse scales 8 (n = 9); predorsal scales 12 – 14 (mean = 13.3, n = 6), first scale (or pair of scales) may be either small and cycloid or same size as others and ctenoid, scales around posterodorsal rim of orbit cycloid or ctenoid, scales reaching anteriorly to above middle of pupil; cheek with 3 rows of cycloid scales, uppermost row of 1 –4 scales, middle row of 7 –8 scales, lowest row of 2 –4 scales; opercle with 3– 4 horizontal rows of 10 –11 mostly ctenoid scales in adults (> 20 mm SL), with 4 –5 in upper row, 3 and 2 in next two rows respectively, and 1 –2 in ventralmost row, which lies below sensory papilla row oi, 3 rows of cycloid scales present in juveniles (<11 mm SL); 3 vertical rows of cycloid scales on pectoral fin base with 3 in anteriormost row, 4 in middle row and 5 in outermost row; 6– 7 (mean = 6.8, n = 4) cycloid scales in midline anterior to pelvic fin base; area between pelvic spine and ventral margin of pectoral fin base, midline of belly and sometimes anteriormost row of body scales beneath axil of pectoral fin base with cycloid scales, other body scales ctenoid. Circumpeduncular scales 12 (n = 5), scales rows in midline between base of last anal ray and first ventral procurrent caudal fin ray 9– 12 (mean = 10.0, n = 5). Upper jaw teeth: outer row of enlarged, slightly curved, regularly spaced canines, decreasing to half the height of teeth at symphysis at end of premaxilla; 2–3 irregular rows of small conical teeth at symphysis grading to a single row at bend and continuing to end of premaxilla. Lower jaw teeth: outer row of enlarged, spaced, curved canines to bend of dentary; 1–2 irregular rows of small conical teeth behind this; innermost row at symphysis half the size of outer row and slightly curved, decreasing in size posteriorly and ending at coronoid process of dentary; single row of tiny conical teeth medial to this from bend of dentary to coronoid process. Tongue rounded with a slight central tip. Gill opening extending anteroventrally to below mid-pupil; gill rakers 4 + 14– 16 = 19– 20 (mean = 4.0 + 15.4 = 19.8, n = 5). Anterior nares in short tube reaching forwards to above anterior margin of upper lip, posterior opening pore-like with slightly raised rim, separated from bony front of orbit by about 4 times pore diameter, nasal sac raised and on anterior onethird of snout. Bony interorbital width equal to pupil diameter; shallowly concave with median fleshy ridge forming broadly rounded W in cross section, a slight groove around posterodorsal margin of eye; epaxialis extending anteriorly to point above anterior third of pupil. Caudal peduncle depth as percentage caudal peduncle length 32.2– 36.6 –40.1 (mean = 35.9, n = 6); head length as percentage SL 28.9– 29.7 –30.10 (mean = 29.5, n = 6); as percentage head length: horizontal eye diameter 33.8– 36.9 (35.7, n = 6); snout length 21.5– 23.3 –25.4 (mean = 23.6, n = 6), cheek depth 13.1– 14.1 –18.5 (mean = 15.8, n = 6). Cephalic sensory papillae as in Fig. 3 View FIGURE 3 , number of papillae in each row given in Table 1. Abdominal/caudal vertebral transition unknown, but presumed to be Type A (inferred from the broad bony interorbital width of> 80% pupil-diameter).
Colour pattern. Live, based on two underwater photographs from Fiji ( Fig. 4 View FIGURE 4 ). Specimen in Figure 4 View FIGURE 4 A with yellowish cheek, light orange snout, nape greenish-yellow with light orange brown mottling, body light orangebrown with rows of half-pupil diameter or larger bright golden- to greenish-yellow spots in centres of most scales, caudal peduncle becoming slightly darker brown in vicinity of ural centrum followed by an amorphous red blotch over bases of caudal fin rays, remainder of caudal rays hyaline, second dorsal fin with reddish tinge. Iris yellow with diagonal purple stripe through pupil and parallel purple stripes dorsally (faint) and ventrally (well developed) at margins of eye. Second specimen ( Fig. 4 View FIGURE 4 B) similar but overall darker, a broad basal yellow stripe in second dorsal and anal fin, stripes in eye dark blue (dorsalmost very faint).
Freshly collected. Similar to above, but yellows and reds not as bright, posterior half of yellow spots with darker border (especially along central scale rows and posteriorly on body), diagonal stripes across eye almost black, basal stripe in second dorsal fin dull orange-red, caudal blotch dull dark red, darker area of peduncle barely apparent ( Fig. 5 View FIGURE 5 ).
Preserved. Overall brownish with scale centres on nape and body lighter, scale pockets margined with combination of deeper, amorphous but rounded brown melanophores which may coalesce to form irregular blotches ( Fig. 6 View FIGURE 6 , green circle), and, more superficially, smaller, darker, and more discretely rounded melanophores ( Fig. 6 View FIGURE 6 , blue circle), with scattered larger dark melanophores on body ( Fig. 6 View FIGURE 6 , red circle), especially on nape to first dorsal fin origin and on abdomen. Scattered melanophores on pectoral fin base, becoming more concentrated dorsally and forming indistinct dark spot on dorsal surface of base ( Fig. 6 View FIGURE 6 , orange ellipse). Opercle and posterodorsal cheek invested with melanophores, remainder of head below eye with only a few pigment cells, tips of jaws with many small dark melanophores. Fins translucent except for vague stripe at base of posterior half of first dorsal fin and along base of second dorsal fin. Caudal peduncle posterior to first procurrent caudal fin rays and onto bases of caudal fin rays darker than area preceding it, forming a diffuse dark caudal blotch. Pigment in caudal fin membranes gradually decreasing distally.
Etymology. Named from the Greek word “finistrini” (φινιστρίνι), a porthole, in allusion to the lines of yellow spots along the sides of the body, reminiscent of the lighted portholes of an ocean liner at night. The common name of “Porthole Pygmygoby” was suggested by Janet Eyre. This species has been informally referred to as T. RW sp. 105. Treated as a noun in apposition.
Distribution. Currently recorded only from the Fiji Islands off the north and north-east coasts of Viti Levu.
Comparisons. The colour pattern of a brownish head and body with golden- to greenish-yellow spots at the centres of most body scales is unique in the genus to T. finistrinum . Described species of Trimma sharing a broad bony interorbital and a dark spot on the hypural region of the caudal peduncle include T. burridgeae Winterbottom, 2016 , T. caudomaculatum Yoshino & Araga, 1975 , T. corerefum Winterbottom, 2016 , T. griffithsi Winterbottom, 1984 , T. hollemani Winterbottom, 2016 , T. nasa Winterbottom, 2005 , T. tevegae Cohen & Davis, 1969 , T. xanthochrum Winterbottom, 2011 , and T. yoshinoi Suzuki et al., 2015 . Trimma griffithsi and T. nasa can be distinguished from T. finistrinum by lacking scales on the cheek, and having only cycloid scales on the nape (vs. cheeks scaled, and ctenoid scales on at least the posterior two-thirds of the nape). The caudal spot in T. griffithsi is much smaller than in any of the other species, and is confined to the lower half of the peduncle. Trimma nasa has a very light colouration with much less pigment on the body compared to the species treated here, except for a dark ‘shadow’ created along the side of the body by pigment on the peritoneum. Trimma xanthochrum and T. yoshinoi differ from T. finistrinum in having the papillae under the eye consisting of short vertical rows of 2–3 papillae each at positions 2, 3 and 4 (vs. single papilla at each of these positions), the mid-snout and posterior interorbital papillae of row p (the third and fifth papillae in the row counting from the anteriormost) consisting of short transverse rows of 2–4 papillae (vs. usually a single papilla), and having branched rays in the mid-region of the pectoral fin (vs. rays unbranched). Trimma finistrinum differs from the remaining five species in having a branched fifth pelvic fin ray. It differ further from T. burridgeae in usually having a shorter second spine of the first dorsal fin, reaching to between base of spine and sixth ray of second dorsal fin (vs. base of fifth ray to mid-peduncle), and more predorsal scales (12–14 vs. 11); and from T. caudomaculatum in usually having a shorter second spine of the first dorsal fin, reaching to between base of spine and sixth ray of second dorsal fin (vs. base of fifth ray to end of peduncle). Further differences between T. finistrinum and T. corerefum include one more pectoral fin ray (14 vs. 12–13), more scales in the predorsal midline (12–14 vs. 10–11), 3 rows of cheek scales (vs. 1–2), more total gill rakers (19–20 vs. 14–18), and 4 or more papillae in rows r and f (vs. 2 each). The new species differs further from T. hollemani in usually having more predorsal midline scales (12–14 vs. 9–12) and total gill rakers (19–20 vs. 17– 19). From T. tevegae it differs in usually having a longer second spine of the first dorsal fin (reaching to the anterior half of the base of the second dorsal fin vs. origin of that fin), and in having more scales in the predorsal midline (12–14 vs. 9–11).
Discussion. A Neighbour-Joining analysis of available COI data for Trimma places the two sequenced specimens of T. finistrinum (T14992, T14993) as phenetically closest to an assemblage within Box A of Winterbottom et al. (2014) containing T. xanthochrum Group 2c ( Palau—possibly the same as T. yoshinoi from Japan), T. gigantum Group 8 ( Palau), T. xanthochrum Group 2a ( Ceram ), T. xanthochrum Group 2 ( Raja Ampat , FakFak and Palawan ), T. xanthochrum Group 2b ( Rabaul ), T. tevegae , T. caudomaculatum , T. burridgeae (as T. tevegae Group 5) and T. hollemani (as T. tevegae Group 4). They are most similar to T. xanthochrum Group 2c ( Palau), but differ from that haplogroup by a minimum of about 15.5% of the base pairs sampled.
The original field notes for ROM 100152 About ROM and ROM 101380 About ROM noted that the collection was made near the wreck of the Nasi Yalodina. Two well separated localities for this wreck were found during a web search. One placement is located almost due north of Tavua, a town at about the middle of the north coast of Viti Levu (e.g. http:// diveadvisor.com/fiji/nasi-yalodina, http://diveseven.com/atlas#lat=-16.827545999918335&lng=177.9860687255 8594&zoom=9, http://www.divebuddy.com/divesite/2433/nasi-yalodina-fiji/), the other lies about 70 km east of this and appears to be the correct positioning for the wreck (https://www.google.com/maps/d/viewer?mid =zgZLcOn4ZK 5M. kLrOIxNNWZ_U).
However, I have omitted the reference to the Nasi Yalodina wreck to avoid future potential confusion regarding the type locality.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |