Erimetopus Rathbun, 1894
|
publication ID |
https://doi.org/10.11646/zootaxa.422.1.1 |
|
DOI |
https://doi.org/10.5281/zenodo.5236801 |
|
persistent identifier |
https://treatment.plazi.org/id/03A88E3D-C202-443B-546D-FC48FE2B7D21 |
|
treatment provided by |
Felipe |
|
scientific name |
Erimetopus Rathbun, 1894 |
| status |
|
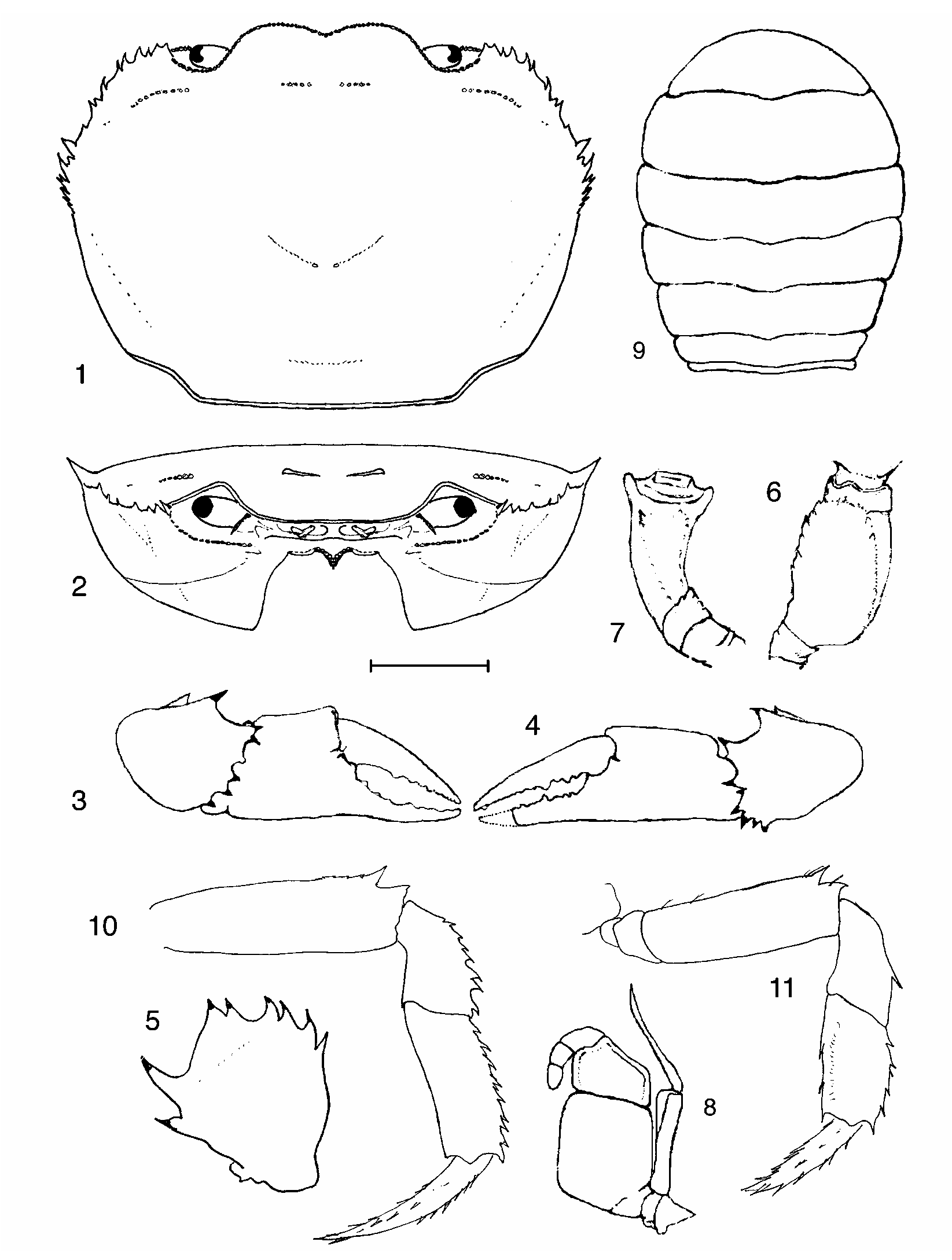
Erimetopus Rathbun, 1894 View in CoL ( Figs. 1–45 View FIGURES 1–11 View FIGURES 12–14 View FIGURES 15–17 View Figures 18–26 View Figures 27–35 View FIGURES 36–38 View FIGURES 39–41 View FIGURES 42–44 View FIGURE 45 )
Erimetopus Rathbun, 1894:26 View in CoL ; 1900: 285; 1905: 270; 1921: 433; Ortmann, 1903: 300; Lenz, 1912: 9; Colosi, 1920: 27; Balss, 1936: 195; Chace, 1942: 225; Cumberlidge, 1999: 45.
Type species. Erimetopus spinosus Rathbun, 1894 View in CoL .
Diagnosis. Carapace outline subhexagonal/rounded; epibranchial tooth large, sharp, pointing forward, positioned well behind postfrontal crest; mandibular palp twosegmented, terminal segment simple; orbit narrow (1/7 cw), upper orbital margin semicircular; anterolateral margin between exorbital and epibranchial teeth very long, curving slightly outward, with several small pointed teeth, lacking identifiable intermediate tooth; exopod of third maxilliped with long flagellum, ischium smooth lacking vertical sulcus; first carpal tooth on inner margin of carpus of cheliped large, slender, pointed, curving forward; series of pointed teeth on outer margin of carpus of P1; superior margins of meri of P2–P5 with two large, pointed distal teeth; carpi of P2–P4 with spines on anterior margins, posterior margins smooth; propodi of P2–P4 with spines on anterior margins, posterior margins smooth; propodus of P5 with spines on anterior and posterior margins.
Discussion. The absence of gonopod characters for some of the taxa included in this study is unfortunate, but it does not in itself discount the validity of utilizing other morphological characters to characterize the genus and to distinguish between its species. For example, our assignment of Erimetopus to the Potamonautidae is based on characters of the mandibular palp (2segmented) ( Bott 1955; Cumberlidge 1999), and of the second antennal segment (broad enough to fill the lateral margin of the antennular fossa). These characters are shared with the other genera of potamonautid freshwater crabs, including Potamonautes . Additional familylevel characters found in Erimetopus (which have still to be confirmed for E. brazzae ) include a first gonopod that is in 4 parts, with a terminal article that is about onethird as long as the subterminal segment ( Figs. 31–33 View Figures 27–35 , 42–44), and a second gonopod with a flagellumlike terminal article ( Figs. 34–35 View Figures 27–35 ).
Fortunately, even without gonopod evidence, Erimetopus is clearly distinct in many respects from other potamonautid genera in Africa. Characters that unequivocally characterize this genus when considered in combination include a carapace outline that is subhexagonal/rounded; a series of pointed teeth on the outer margin of the carpus of the cheliped; two large, pointed distal teeth on the superior margins of the meri of pereopods P2–P5; spines on the anterior margins of the carpi of pereopods P2–P4; spines on the anterior margins of the propodi of pereopods P2–P4; and spines on the anterior and posterior margins of the propodus of P5. Similarly, other characters of the carapace, eyes, and pereopods easily distinguish between the two species of Erimetopus .
The absence of gonopod evidence in this case presents a challenge to taxonomists who are continually seeking new characters (whether gonopod or not) to distinguish between taxa. In this regard, it should be remembered that gonopod characters (although informative) restrict the use of identification keys to males, whereas diagnostic characters of the carapace, eyes, anterior sternum, and pereopods offer the advantage that they permit the identification of all specimens, including females and juveniles.
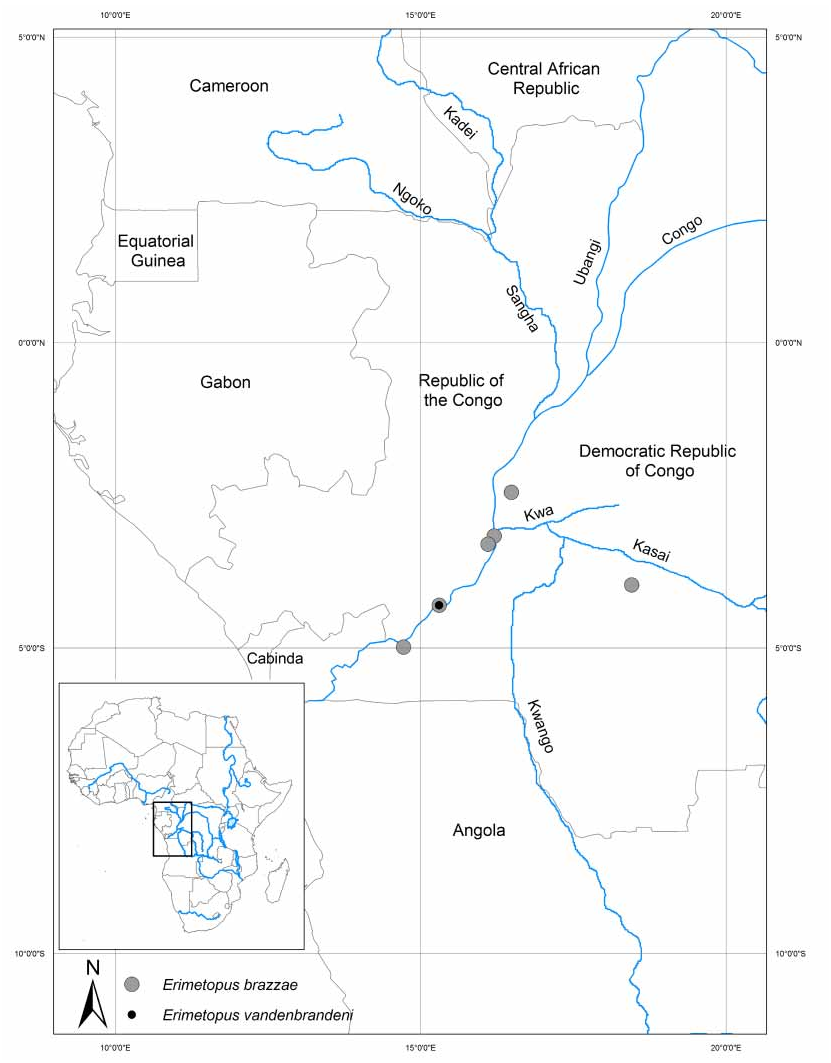
The lack of gonopod evidence for E. brazzae prompted Bott (1955) to admit to uncertainty about the proper taxonomic placement of Erimetopus . Despite this uncertainty, that author took the radical taxonomic step of assigning Erimetopus to the genus Potamonautes . Bott (1955) assigned E. brazzae to the genus Potamonautes because both taxa share a 2segmented mandibular palp, and because both lack an intermediate tooth on the anterolateral margin of the carapace between the exorbital and epibranchial teeth. However, neither of these characters is exclusive to Potamonautes or to Erimetopus . For example, a 2segmented mandibular palp is shared by all African potamonautid genera, and an intermediate tooth is also lacking in Potamonemus (Cumberlidge 1999) . Unfortunately, the characters used by Bott (1955) to distinguish E. brazzae from his subgenera of Potamonautes (such as differences in the cornea length and carapace anterolateral margin tooth patterns) are not shared by all species of Erimetopus , and are therefore interpreted here as characters suitable for species separation within the genus Erimetopus . In the present work, characters that are shared by both species of Erimetopus , and which distinguish this genus from Potamonautes include a subhexagonal carapace outline, the presence of a row of small teeth on the anterolateral margin between the exorbital and epibranchial teeth, 2 or 3 teeth on the outer margin of the carpus of the cheliped, and spines on the margins of the carpi and propodi of pereopods P2–P5.
In the present study we have made a cautious use of the newly available gonopod characters of E. vandenbrandeni , because of the absence of male specimens of E. brazzae , E. spinosus and P. (E.) b. frontospinulosa. As a general rule, certain gonopod characters tend to be invariant at different taxonomic levels. For example, a fourpart first gonopod (consisting of three segments plus a welldeveloped terminal article) is typical of that found in all families of Old World freshwater crabs ( Bott 1970; Ng 1988; Cumberlidge 1999). Gonopod characters that tend to be invariant among congeners include the overall length and shape of the subterminal segments and terminal articles of gonopods 1 and 2. We consider it likely that these characters in E. vandenbrandeni will likely prove to be similar to those of E. brazzae ( Bott 1955; Cumberlidge 1999) when the appropriate specimens become available.
Relationships. A number of characters seen in Erimetopus are also found in species of platythelphusid African freshwater crabs such as Platythelphusa armata (A. Milne Edwards, 1887) from Lake Tanganyika in East Africa (Cumberlidge et al. 1999). These characters include a subhexagonal carapace outline, a row of small teeth on the anterolateral margin of the carapace between the exorbital and epibranchial teeth, a cheliped whose carpus has 2 or 3 teeth on the outer margin, and a front whose anterior margin projects straight out or is only slightly deflexed. However, Erimetopus can be easily distinguished from Platythelphusa as follows. The number of segments of the mandibular palp is normally used as a familylevel character for the freshwater crabs ( Bott 1970; Ng 1988; Cumberlidge 1999). In this case, the mandibular palp of Platythelphusa is 3segmented, whereas that of Erimetopus is 2segmented. In addition, the following characters in Platythelphusa are different from those in Erimetopus : the external angles of the front are either marked by sharp spines or by small granules, there is a stout triangular process (which may be produced into a small tooth) beneath the external angles of the front that descends into the orbital hiatus, and the medial end of the suborbital margin is marked by a distinct spine or small tooth (Cumberlidge et al. 1999). In addition, the first gonopod of Platythelphusa tapers to a pointed tip, whereas that of Erimetopus is tubular (although this character has yet to be confirmed for E. brazzae ).
Several workers (A. MilneEdwards 1886; Balss 1936; Bott 1970; Rodriguez 1982, 1986; Ng & Rodriguez 1995) have commented on similarities (such as the subhexagonal/ rounded carapace outline and the bilobed frontal margin) between E. brazzae and species of South American river crabs of the family Trichodactylidae (such as Trichodactylus and Dilocarcinus ). However, we consider that such similarities are insufficient to argue for a relationship between Erimetopus and the trichodactylids. Moreover, there are a number of major differences in the diagnostic characters of Erimetopus and members of the Trichodactylidae ( Rodriguez 1992; Magalhães & Türkay 1996a,b,c) which strongly discount a close relationship between these taxa. For example, the mandibular palp of Erimetopus is 2segmented, while that of the trichodactylids is 3segmented; the merus of the third maxilliped of Erimetopus is broadly rectangular, while that of the trichodactylids is slim and triangular; and the dactyli of P2–P5 of Erimetopus have rows of stiff corneous spines, while those of the trichodactylids lack spines and are fringed with hairlike setae. In addition, gonopod 1 of Erimetopus is 3segmented with a distinct terminal article, while that of the trichodactylids is 3segmented, and lacks the terminal article. These conclusions are supported by a number of substantial studies that indicate that the trichodactylids belong to a lineage that is independent of any of the other freshwater crab families (including the African Potamonautidae ), and that the Trichodactylidae properly belongs in, or close to, the marine superfamily Portunoidea ( Rodriguez 1992; Magalhães & Türkay 1996a,b,c; Sternberg 1997, 1998; Sternberg et al. 1999; Sternberg & Cumberlidge 2001, 2003; Martin & Davis 2001).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Brachyura |
|
SuperFamily |
Potamoidea |
|
Family |
Erimetopus Rathbun, 1894
| Cumberlidge, Neil & Reed, Sadie K. 2004 |
Erimetopus
| Chace, F. A. 1942: 225 |
| Balss, H. 1936: 195 |
| Rathbun, M. J. 1921: 433 |
| Colosi, G. 1920: 27 |
| Lenz, H. 1912: 9 |
| Rathbun, M. J. 1905: 270 |
| Ortmann, A. E. 1903: 300 |
| Rathbun, M. J. 1900: 285 |
| Rathbun, M. J. 1894: 26 |