Tridentata borneensis ( Billard, 1925a )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5428.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:041905ED-FCED-4ED5-8248-E9AA8D6271E9 |
|
DOI |
https://doi.org/10.5281/zenodo.10870318 |
|
persistent identifier |
https://treatment.plazi.org/id/03A5566C-FFDC-FFA1-FF1D-FADB2BC4FA23 |
|
treatment provided by |
Plazi |
|
scientific name |
Tridentata borneensis ( Billard, 1925a ) |
| status |
|
Tridentata borneensis ( Billard, 1925a)
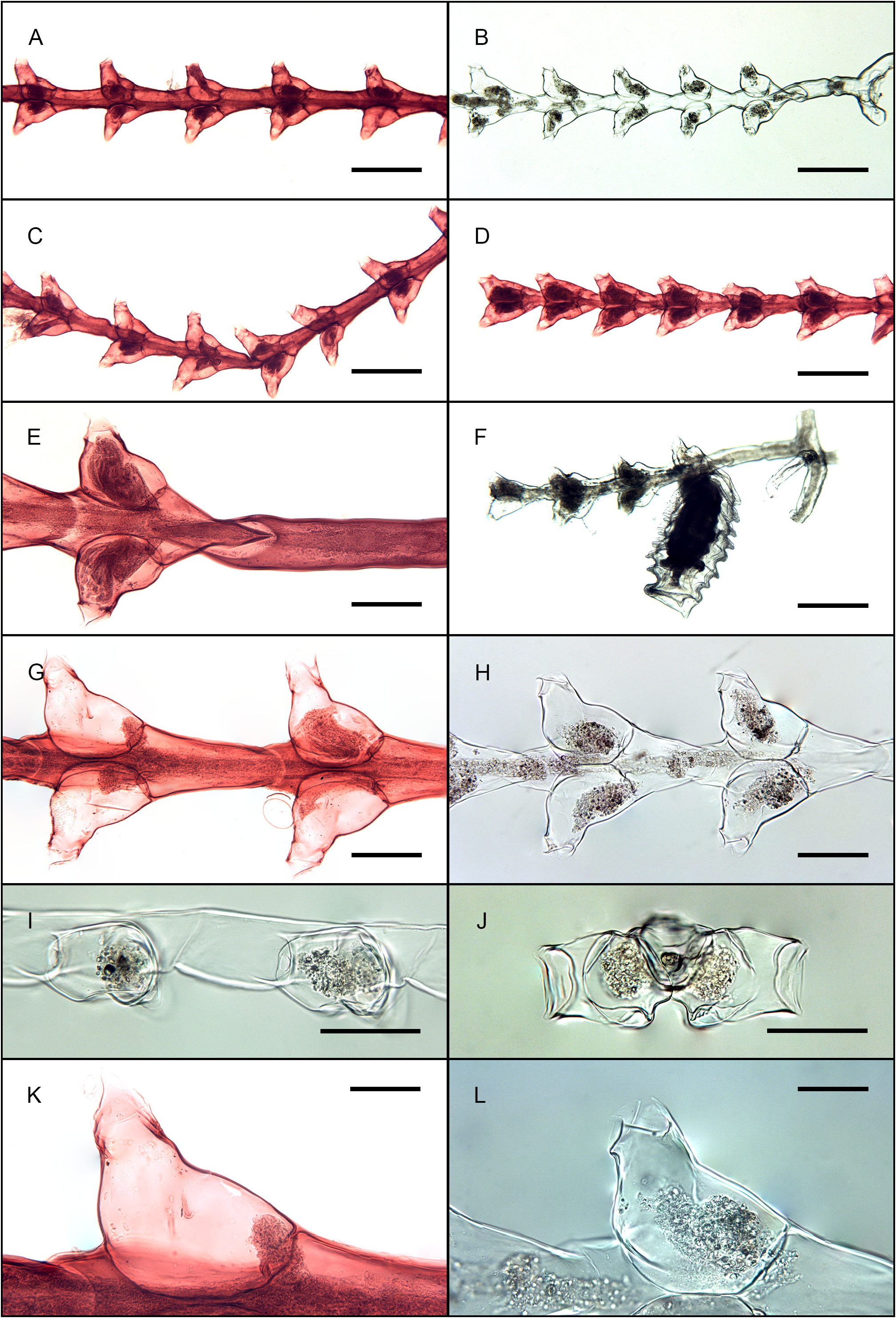
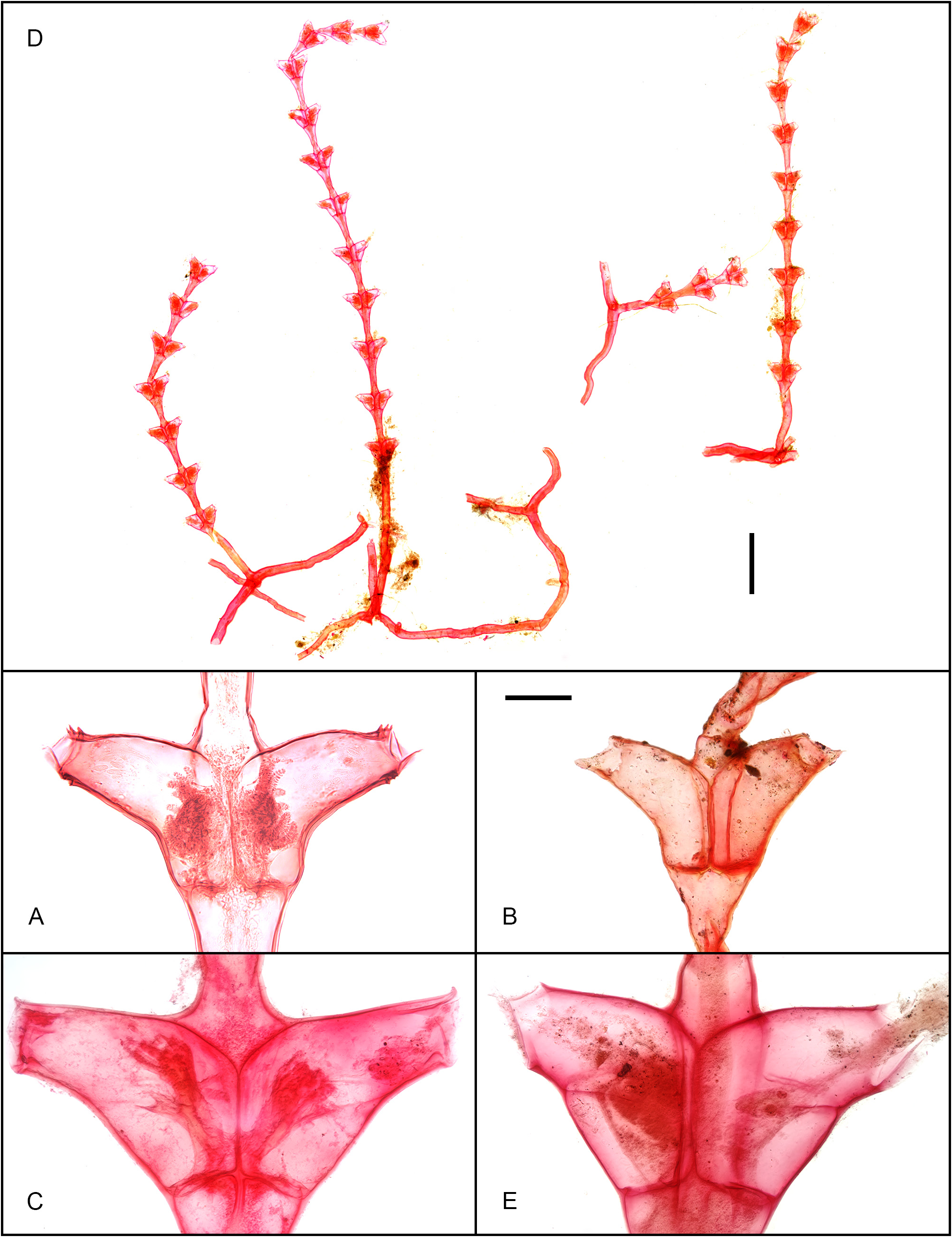
Figs. 15 View FIGURE 15 , 18A–C View FIGURE 18 , 28 View FIGURE 28
Sertularia borneensis Billard, 1925a: 649 View in CoL , fig. 1D.— Billard, 1925b: 171, fig. 31.— Pennycuik, 1959: 197, pl. 6, fig. 5.— Van Soest, 1976: 84.— Van Praët, 1979: 900.— Vervoort & Vasseur, 1977: 63, figs 26b, 27b.— Gibbons & Ryland, 1989: 418, fig. 34.— Preker, 2001: 154.— Schuchert, 2003: 189, fig. 43.— Preker & Lawn, 2010: 131.— Preker & Lawn, 2012: 52, fig. 12.
Tridentata borneensis —? Kirkendale & Calder, 2003: 176.— Calder et al., 2022: 590 View Cited Treatment , fig. 6G–I.
Sertularia malayensis sorongensis Leloup, 1930: 3 , figs 1‒2, pl. 1 fig. 1.
Sertularia vervoorti Migotto & Calder, 1998: 170 View in CoL , figs 1–3, syn. nov.
Tridentata vervoorti — Calder & Kirkendale, 2005: 486.
Tridentata longa — Calder et al., 2003: 1194, fig. 15.— Calder et al., 2022: 591 [non Sertularia linealis var. longa Millard, 1958: 197 View in CoL , fig. 8E = Tridentata longa ( Millard, 1958) ].
Sertularia turbinata View in CoL — Vervoort & Vasseur, 1977: 60, figs 26a, 27a [non Dynamena turbinata Lamouroux, 1816: 180 View in CoL = Tridentata turbinata ( Lamouroux, 1816) View in CoL ].
Sertularia westindica View in CoL — Cooke, 1975: 100, pl. 5 fig. 1.— Tang, 1991: 28, fig. 3 [non Tridentata westindica Stechow, 1919: 38 View in CoL , fig. 5 = Tridentata turbinata ( Lamouroux, 1816) View in CoL , see below].
Sertularia west-indica View in CoL — Mammen, 1965: 40, fig. 71 (subsequent incorrect spelling).
? Sertularia tumida View in CoL — Galea, 2008: 36 View Cited Treatment , fig. 7A (non Sertularia tumida Allman, 1877: 23 View in CoL , pl. 16 figs 3–4).
non Sertularia borneensis View in CoL f. parvula Vannucci, 1949: 249, pl. 3 figs 47–48 [= Tridentata turbinata ( Lamouroux, 1816) View in CoL ].
Material examined. Holotype: MNHN H.L.709, Indonesia, Borneo Bank, Siboga Stn. 80, -2.4166°, 117.7166°, 34 m, 13 Jun 1899, microslide preparation belonging to the syntype series, comprising 3 sterile stems growing on Macrorhynchia phoenicea ; this is one of the two slides 8 housed in MNHN of Paris that was examined, and contains one stem arising from a portion of hydrorhiza, the proximal portion of a second stem detached immediately above its origin from hydrorhiza, and the distal portion of a third stem. Additional material: MSNMCoe370, Indonesia, Bali, Pemuteran, -8.144146°, 114.657994°, 0‒0.5 m, 01 Apr 2023, a profuse, fertile colony (with stems reaching 9 mm in height) partly growing on ship hull, and partly on stems on M. philippina growing themselves on the same hull, GenBank: OR872071 (16S). Comparative material: NHM 23.2.15.46 (designated here as lectotype) and NHM 23.2.15.47 (designated here as paralectotype), Republic of Maldives, Huvadhu Atoll, 57 m, two microslides as parts of the syntype of Thuiaria 9 maldivensis Borradaile, 1905 , each showcasing a single stem ( Fig. 16A View FIGURE 16 herein).—MNHN-IK-2012-16518, French Polynesia, Marquesas, MUSORSTOM 9, Stn. CP1265, colony of Dynamena heterodonta ( Jarvis, 1922) , with sterile stems, up to 2.6 cm high ( Galea 2016: 6).— ZSM 20041646, locality unknown [“possibly Indian Ocean” ( Stechow 1926: 105)], holotype of Tridentata occulta Stechow, 1926 , microslide with a “tiny piece” of colony ( Ruthensteiner et al., 2008: 24) ( Fig. 16C View FIGURE 16 herein).—MHNG-INVE-91120, French Lesser Antilles, Martinique, Le Vauclin, Pointe Faula, 23 Feb 2014, fertile colony of Amphisbetia distans ( Lamouroux, 1816) on floating Sargassum sp. [see Galea & Ferry (2015: 234)].— ZSM 20041550, Kingom of Tonga, leg. Kirchenpauer, holotype of Sertularia tongensis Stechow, 1919b , microslide with a “small piece” of colony ( Ruthensteiner et al., 2008: 24) ( Fig. 16D View FIGURE 16 herein).— ZSM 20050705 & 20050706, French Lesser Antilles, Martinique, Ste. Anne, 03 Apr 1898, leg. F. Doflein, holotype of Tridentata westindica Stechow, 1919a , two microslides with “colony pieces” ( Ruthensteiner et al., 2008: 24) ( Figs 16F View FIGURE 16 , 17D View FIGURE 17 herein).—HRG-0342, French Lesser Antilles, Les Saintes, Terrede-Haut, 27 Mar 2008, colony of Tridentata turbinata ( Lamouroux, 1816) composed of several sterile stem, up to 4 mm high, on Dictyota sp. ( Galea 2008: 37).
Remarks. For contemporary descriptions of Tr. borneensis , refer to Gibbons & Ryland (1989) and Schuchert (2003) (both as Sertularia ). The species appears genetically divergent from all its congeners, with which it nevertheless forms a monophyletic clade, exclusive of Tr. loculosa ( Fig. 28 View FIGURE 28 ).
Morphologically, it should be added that its hydrothecal walls are distinctively chiseled, especially when observed laterally ( Figs 15I View FIGURE 15 , 18C View FIGURE 18 ) or apically ( Figs 15J View FIGURE 15 , 18B View FIGURE 18 ), with a broad, transverse constriction in middle part and an arching ridge running parallel to the adnate adaxial wall ( Figs 15K–L View FIGURE 15 , 18A View FIGURE 18 ). These features are easily overlooked in specimens retaining their coenosarc, unless the stems are carefully examined in lateral view (and at a high magnification of the stereomicroscope). Ideal observations are made on material cleared with a 1‒2% solution of domestic bleach. Sole Pennycuik (1959: 197, pl. 6 fig. 5, left-hand side drawing) was able to notice “[o]ne detail which [Billard] failed to observe”, namely “the well developed tooth to which the abcauline caecum of the hydranth is attached” (see Figs 15K View FIGURE 15 , 18A View FIGURE 18 herein).
The best illustration of a fully-formed gonotheca so far is that of Gibbons & Ryland (1989: fig. 34D). Calder et al. (2022: fig. 6I) also provided a good figure, and a new illustration is now available in Fig. 15F View FIGURE 15 herein. Mammen (1965: fig. 71, as S. west-indica ) and Schuchert (2003: fig. 43C, as S. borneensis ) obviously had specimens with immature gonothecae whose distal horns were not yet formed. It should be noted that the latter author misinterpreted the outer ornamentation of the gonothecal wall (“wall with sharp, projecting spiral structure in 7‒8 loops”), the ridges being irrefutably transverse in this species.
The specific name became available in 1925, not 1924, as mentioned in several earlier accounts (e.g. Billard 1925b; Van Soest 1976; Vervoort & Vasseur 1977; Van Praët 1979; Gibbons & Ryland 1989; Preker 2001; Preker & Lawn 2010). Indeed, Billard’s report was read at the December 23 th, 1924 meeting of the French Zoological Society, and published subsequently on April 10 th, 1925 in numbers 8‒10 of its Bulletin.
As noted by Calder (1991: 110), “[t]he hydroid identified by Vannucci (1949) as Sertularia borneensis f. parvula appears to have had an intrathecal septum, and it is referred […] to T[ridentata] turbinata ( Lamouroux, 1816) ”, and we agree.
We also concur with Gibbons & Ryland’s (1989: 419), Calder’s (1991: 110, 2020: 221) and Schuchert’s (2003: 190) arguments that Vervoort & Vasseur’s (1977) specimens assigned to Tr. turbinata ( Lamouroux, 1816) belong to the present species instead.
The scanning electron microscope images provided by Migotto & Vervoort (1998: figs 2‒3) of their S. vervoorti leave no doubt about its conspecificity with the present hydroid. Their material obviously bore immature gonothecae.
The Guam record by Kirkendale & Calder (2003: 176) is unreliable in the absence of a formal description and/or illustrations. Their colonies are reportedly said “[r]igid, linear […], with upright cormoids perpendicular to the substratum and usually not more than ⁓ 3 cm in height”, a size not reported so far in Tr. borneensis [literature data indicate stems ranging commonly from “about 1 cm ” ( Billard 1925b: 55; present study), to 1.3 cm ( Gibbons & Ryland 1989: 418), to 1.5 cm ( Preker & Lawn 2010: 131), to maximum 2 cm ( Schuchert 2003: 189)]. In addition, for the synonymy of this species, the authors referred to Calder (1991), who considered it as a junior synonym of Tr. tumida ( Allman, 1877) , which further complicates the taxonomic assessment of their material. Calder et al. (2022: 592), however, listed this record in the reported distribution of Tr. borneensis .
The material from Guadeloupe assigned to S. tumida by Galea (2008: 36) may belong to the present species, as well. Unfortunately, the specimen used to draw fig. 7A in that report is now lost, but photomicrographs of it are still available, though of limited value, as the hydrothecae are only seen in frontal view.
Calder et al. (2022: 591) noted that the “report of S[ertularia] longa from the Galápagos Islands by Calder et al. (2003, as T[r]. longa ), based on sterile specimens, is believed […] to have been based on T[r]. borneensis instead”, and we concur.
One of the two slides deposited at the MNHN of Paris, belonging to the syntype of Tr. borneensis , was reexamined. Although the mounted stems could only be seen frontally, the outer, arched hydrothecal ridge (running parallel to the adnate portion of the adaxial wall) is well visible in some hydrothecae ( Fig. 15G, K View FIGURE 15 ), as is the internal perisarc projection for the attachment of the blind sac ( Figs 15K View FIGURE 15 , 18A View FIGURE 18 ). There is no doubt that the type material of Tr. borneensis exhibits the same morphological features as those met with in the new material from Bali.
The taxonomic status of Tr. borneensis was a matter of debate for nearly a century. Many authors [e.g. Billard (1925b: 172‒173); Mammen (1965: 41); Cooke (1975: 100); Calder (1991: 109‒110; 2020: 220‒221); Schuchert (2003: 189‒190); Calder et al. (2022: 591)] noted morphological similarities with one or several nominal species, namely: S. tumida Allman, 1877 , Thuiaria maldivensis Borradaile, 1905 , S. tongensis Stechow, 1919b , Tr. westindica Stechow, 1919a , S. malayensis var. sorongensis Leloup, 1930 .
According to Calder (1991: 110), type material of S. tumida could not be located, but he subjectively assigned a Bermudan hydroid to that species on the account of some apparent morphological similarities 10. The original account on S. tumida is, unfortunately, little informative (succinct description, sketchy illustrations) to allow a reliable identification, and Allman’s nominal species may possibly have been based on other hydroids known to occur in the (tropical) western Atlantic, e.g. Amphisbetia distans ( Lamouroux, 1816) [ Calder (1991), as Tr. distans ; Migotto (1996), as S. distans ; Galea & Ferry (2015), as S. distans ], Tr. turbinata ( Lamouroux, 1816) 11 [ Migotto (1996), as S. turbinata ; Galea & Ferry (2015), as S. turbinata ], and Dynamena dalmasi ( Versluys, 1899) [ Vervoort (1959), as S. dalmasi ; Calder (2013); Galea et al. (2021)]. The illustration provided by Allman (1877: pl. 16 fig. 4) suggests, at least, that S. tumida has longer and slenderer internodes compared to Tr. borneensis , and it is unlikely to be a senior synonym of it.
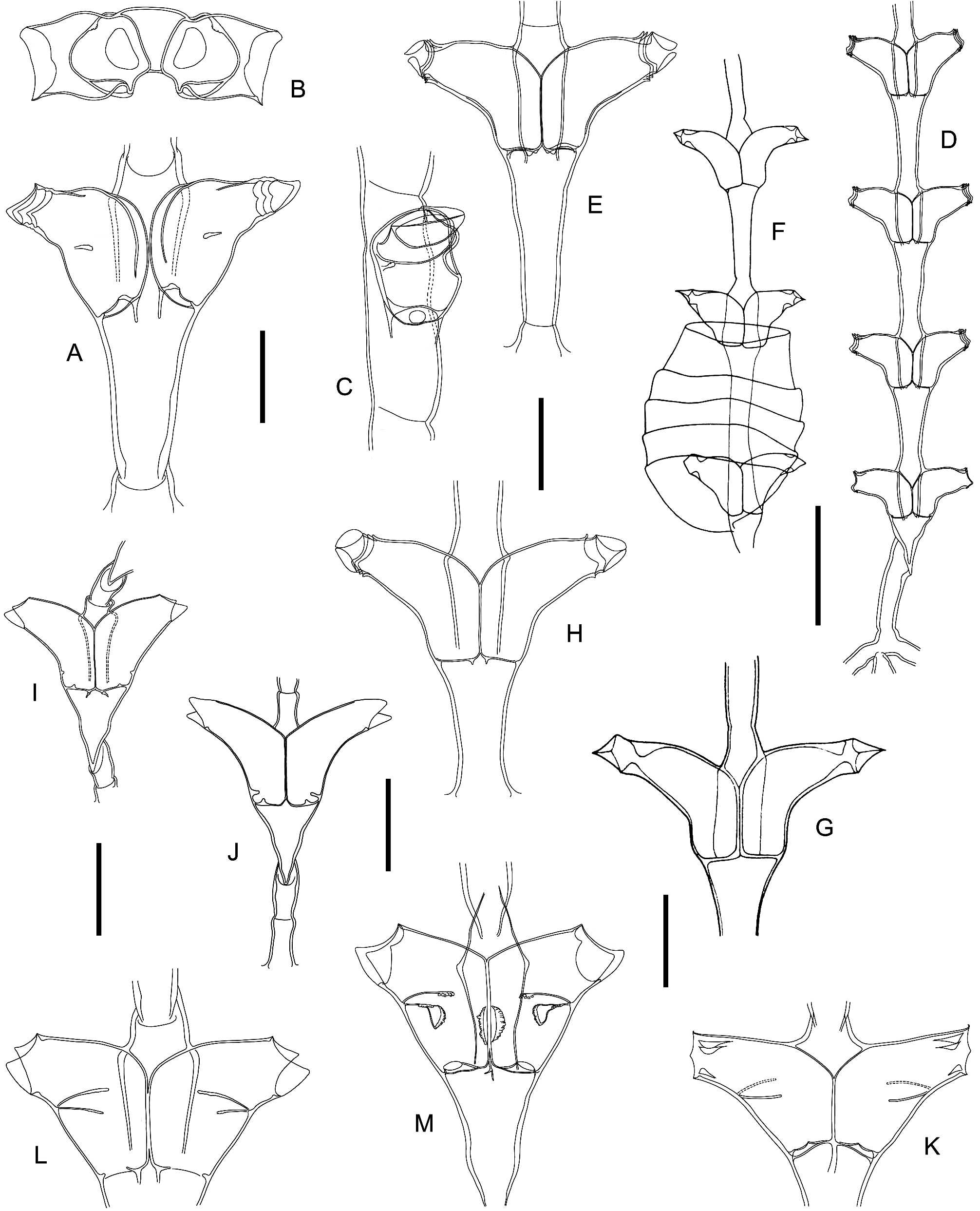
Type material of Th. maldivensis was reexamined ( Figs 16A–B View FIGURE 16 , 17A View FIGURE 17 , 18D–E View FIGURE 18 ) and, as originally reported, both stems mounted on slides are unfortunately sterile. Specimen NHM 23.2.15.46 is complete ( Fig. 16A View FIGURE 16 , left), and it is here designated as lectotype; it originates from a branched stolon and is composed proximally of a smooth, 390 µm long, athecate part, ending in an oblique hinge joint, and a 5.15 mm long, distal part divided into a regular sequence of thecate internodes; the nodes are faint and transverse, 70‒85 µm wide, and the internodes 540‒675 µm long, rather slender, though gradually expanding from their proximal to distal ends, and there merging smoothly into a pair of contiguous hydrothecae; the latter are slightly more than half adnate (adnate part 180‒235 µm long, free part 150‒180 µm long), almost tubular (maximum width 135‒150 µm), though curving sharply away from the internode, more markedly on their abcauline side (proximally on stem, the angle is of 70°, but decreases distally to 50°; the abcauline wall is 195‒240 µm long). The aperture, whose diameter is 80‒90 µm, comprises 2 large, lateroabcauline, triangular cusps with rounded tips, in addition to a minute adcauline cusp. The hydranths (all retracted into their corresponding thecae) are not satisfactorily preserved, and do not seem to possess an abcauline caecum. Though difficult to observe, the opercular apparatus is composed of a large abcauline flap, and what appears now to be 2 latero-adcauline, triangular flaps, the latter possibly resulting from the shredding of an original, pleated flap (upon the chemical treatment used to prepare the mounts).
Gravier-Bonnet & Bourmaud (2012: 110, as S. maldivensis ) included a putative new record of this species from the Maldives but did not provide a description and/or illustrations of this poorly-documented species, simply stating that “[f]ertile specimen (sic) is needed to go further in identification”. The record from Hawaii by Calder (2020: 220, fig. 6C–E, as Tr. maldivensis ), is clearly a misidentification, as the hydrothecae do not curve rapidly away from their internodes.
The available, scant information on Th. maldivensis appears, at first, potentially problematic. However, the apparent lack of an abcauline caecum would place it into the genus Dynamena Lamouroux, 1812 , where it comes close to D. heterodonta ( Jarvis, 1922) . Type material of the latter ( Fig. 18F, G View FIGURE 18 ) was reexamined by Vervoort & Vasseur (1977: 39) 12, and the specimens from the Marquesas studied by Galea (2016: 6, fig. 1L, N) appear also very similar ( Fig. 18H View FIGURE 18 ).
Hydrothecae of Th. maldivensis , through their distinctive abcauline bent, recall from afar those of Tr. malayensis ( Billard, 1925a) , but their free part in that species is comparatively longer, and the marginal cusps are much sharper [ Vervoort & Vasseur (1977: fig. 25C‒D), type material]. The quite unusual specimens of Amphisbetia distans ( Lamouroux, 1816) depicted by Calder (1991: fig. 55A‒B, as Tr. distans ) have similar hydrothecae, but the division of the stem into internodes is typical of Lamouroux’ hydroid [“Occasionally two thecate internodes separated by a short athecate internode marked by an oblique node at proximal end and an oblique hinge-joint at distal end” ( Calder 1991: 106); see also below the note on the taxonomic status of Tr. occulta Stechow, 1926 ].
No additional nominal species of sertulariids are known to have hydrothecae resembling those of Th. maldivensis . Therefore, Th. maldivensis and D. heterodonta are very likely coterminous. Consequently, Borradaile’ (1905) species has priority over that created by Jarvis (1922), and it is here referred to as D. maldivensis ( Borradaile, 1905) , comb. nov., due to the absence of an abcauline caecum in the polyps. It should also be noted that the type localities of the two nominal species are both situated in the western part of the Indian Ocean [Huvadhu Atoll, the Republic of Maldives for Th. maldivensis , and Saint Brandon (Cargados Carajos Shoals), Mauritius, for D. heterodonta ], being distant of 2400 km.
Type materials of three nominal species created by Stechow, namely S. tongensis ( Fig. 16D View FIGURE 16 ), Tr. westindica ( Fig. 16F View FIGURE 16 , 17D View FIGURE 17 ), as well as the rather enigmatic taxon (not illustrated so far) Tr. occulta ( Fig. 16C View FIGURE 16 ), were also examined.
The hydrothecae of S. tongensis show the same shape, submarginal intrathecal perisarc projections (two latero-adcauline, one abcauline), and horseshoe-shaped internal ridge ( Figs 16E View FIGURE 16 , 17C View FIGURE 17 , 18K View FIGURE 18 ) as S. orthogonalis Gibbons & Ryland, 1989 , the latter evidently becoming a junior synonym of Stechow’ species (N.B.: the type locality of the former, the Kingdom of Tonga, is distant of only about 800 km from that of the latter, the Fiji Islands). Although originally described based on infertile stems, a description of its gonothecae should be based on Gibbons & Ryland’s account. The paired condition of its hydrothecae, their three-cusped margin and the transversely-ridged gonothecae justify the assignment of Stechow’s hydroid to the genus Tridentata Stechow, 1919a , under the binomen Tr. tongensis ( Stechow, 1919b) , a combination introduced earlier by Calder (1991: 110). In an earlier paper, one of us ( Galea 2010: 18–20) considered Sertularella tongensis Stechow, 1919 as belonging to the genus Sertularia Linnaeus, 1758 , on the account of the morphology of its closing apparatus. In doing so, it became a secondary homonym of the present hydroid, for which the replacement name S. ephemera Galea, 2010 was introduced. However, molecular evidence led Song et al. (2019) create a new genus, Bicaularia , for it. Consequently, S. ephemera should be now dropped. The geographical distribution of S. tongensis is restricted, to date, to the Kingdom of Tonga ( Stechow 1919b, as S. tongensis ; Gibbons & Ryland 1989, as S. orthogonalis ) and the Hawaiian Islands ( Calder & Faucci 2021, as Tr. orthogonalis ).
Tridentata westindica has hydrothecae provided with a transverse, horseshoe-shaped, internal ridge in their middle part ( Figs 16G View FIGURE 16 , 17E View FIGURE 17 , 18L View FIGURE 18 ), and are indistinguishable from those of the type of Tr. turbinata ( Lamouroux, 1816) depicted by Billard (1925b: fig. 34M, as S. turbinata ). Tridentata westindica is thus a junior synonym of Lamouroux’ species. The occurrence of Tr. turbinata in Martinique, its type locality, is confidently-documented ( Galea & Ferry 2015, as S. turbinata ). Based on specimens from Les Saintes (HRG-0342), whose hydrothecae have been perfectly cleared with domestic bleach, it is realized that there are many attachment sites of the hydranths to the inner walls of their corresponding hydrothecae, as illustrated in Fig. 18M View FIGURE 18 .
The shape of the hydrothecae of Tr. occulta , together with the characteristic presence of internal, perisarcal projections ( Figs 17B View FIGURE 17 , 18I View FIGURE 18 ), as well as the division of the stem into moderately-long, hydrothecate internodes occasionally alternating with comparatively-shorter, athecate internodes (the latter with a proximal, transverse node and a distal, oblique node; Figs 16C View FIGURE 16 , 18I View FIGURE 18 ), are features of Amphisbetia distans ( Lamouroux, 1816) ( Billard 1925c: fig. 1, as S. distans ; N.B.: his figs 1F‒G illustrate type material). Consequently, T. occulta becomes a junior synonym of Lamouroux’ species.
In conclusion, Tr. borneensis has only two junior synonyms: an objective one, namely S. vervoorti , and a subjective one, S. malayensis var. sorongensis (specimens of the latter should be examined to confirm the synonymy). As demonstrated above, none of the nominal species whose basionyms are S. tumida, Th. maldivensis , S. tongensis and Tr. westindica belong to its synonymy.
Distribution. Indonesia ( Billard 1925a, b, as S. borneensis ; Leloup 1930, as S. malayensis sorongensis ; Schuchert 2003, as S. borneensis ; present study), India ( Mammen 1965, as S. west-indica ), Spratly Islands ( Tang 1991, as S. westindica ), Philippines ( Gibbons & Ryland 1989, as S. borneensis ), Australia ( Pennycuik 1959, as S. borneensis ; Preker 2001, as S. borneensis ; Preker & Lawn 2010, as S. borneensis ), possibly Guam ( Kirkendale & Calder 2003), Marshall Islands ( Cooke 1975, as S. westindica ), Fiji ( Gibbons & Ryland 1989, as S. borneensis ), French Polynesia ( Vervoort & Vasseur 1977, as S. turbinata ), Galápagos ( Calder et al. 2003, as S. longa ), Cocos Island ( Calder et al. 2022), Brazil ( Migotto & Calder 1998, as S. vervoorti ), Caribbean coast of Panama ( Calder & Kirkendale 2005, as Tr. vervoorti ), possibly French Lesser Antilles ( Galea 2008, as S. tumida ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Hydroidolina |
|
Order |
|
|
Family |
|
|
Genus |
Tridentata borneensis ( Billard, 1925a )
| Galea, Horia R. & Maggioni, Davide 2024 |
Tridentata vervoorti
| Calder, D. R. & Kirkendale, L. 2005: 486 |
Tridentata borneensis
| Calder, D. R. & Carlton, J. T. & Keith, I. & Ashton, G. V. & Larson, K. & Ruiz, G. M. & Herrera, E. & Golfin, G. 2022: 590 |
| Kirkendale, L. & Calder, D. R. 2003: 176 |
Tridentata longa
| Calder, D. R. & Carlton, J. T. & Keith, I. & Ashton, G. V. & Larson, K. & Ruiz, G. M. & Herrera, E. & Golfin, G. 2022: 591 |
| Calder, D. R. & Mallinson, J. J. & Collins, K. & Hickman, C. P. 2003: 1194 |
| Millard, N. A. H. 1958: 197 |
Sertularia vervoorti
| Migotto A. E. & Calder, D. R. 1998: 170 |
Sertularia turbinata
| Vervoort, W. & Vasseur, P. 1977: 60 |
| Lamouroux, J. V. F. 1816: 180 |
Sertularia west-indica
| Mammen, T. A. 1965: 40 |
Sertularia borneensis
| Vannucci, M. 1949: 249 |
Sertularia malayensis sorongensis
| Leloup, E. 1930: 3 |
Sertularia borneensis
| Preker, M. & Lawn, I. D. 2012: 52 |
| Preker, M. & Lawn, I. D. 2010: 131 |
| Schuchert, P. 2003: 189 |
| Preker, M. 2001: 154 |
| Gibbons, M. J. & Ryland, J. S. 1989: 418 |
| Van Praet, M. 1979: 900 |
| Vervoort, W. & Vasseur, P. 1977: 63 |
| Van Soest, R. W. M. 1976: 84 |
| Pennycuik, P. R. 1959: 197 |
| Billard, A. 1925: 649 |
| Billard, A. 1925: 171 |