Corymorpha balssi Stechow, 1932
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5428.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:041905ED-FCED-4ED5-8248-E9AA8D6271E9 |
|
DOI |
https://doi.org/10.5281/zenodo.10870312 |
|
persistent identifier |
https://treatment.plazi.org/id/03A5566C-FFCF-FFB1-FF1D-FED82EC7FC13 |
|
treatment provided by |
Plazi |
|
scientific name |
Corymorpha balssi Stechow, 1932 |
| status |
|
Corymorpha balssi Stechow, 1932 View in CoL
Figs 1–8 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 , 23 View FIGURE 23 ; Table 1 View TABLE 1
Corymorpha (Euphysa) balssi Stechow, 1932: 82 View in CoL .— Ruthensteiner et al., 2008: 13, figs 3E, 7B.— Watson, 2008: 187.
? Euphysora bitungensis Xu, Huang & Guo View in CoL (in Lin et al.), 2013: 248, figs 4, 8.
? Euphysora juliephillipsi Gershwin et al., 2010: 59 View in CoL , fig. 1E–F.
Material examined. Type material: ZSM 20040176: Australia, Western Australia, Shark Bay, NNE of Heirisson Prong, 11–12.5 m, 18 Jun 1905, ethanol-preserved colonies on Schizophrys dama (Herbst, 1804) .— ZSM 20041647– 20041651, 5 microslide preparations with detached polyps. Additional material: MSNMCoe375: Indonesia, Bali, Amed, dive site known as “Ghost Bay”, -8.332965°, 115.643044°, 20 m, 25 Apr 2023, four formalin-fixed crabs ( Hyastenus sp. ) with polyps on their carapace.—MSNMCoe376: Indonesia, Bali, Tulamben, dive site known as “Seraya”, -8.295692°, 115.612838°, 18–22 m, 23 Apr 2023, one formalin-fixed crab ( Hyastenus sp. ) with polyps on its carapace.—MSNMCoe377: Indonesia, Bali, Amed, dive site known as “Melasti”, -8.330810°, 115.639825°, 18– 20 m, 20 Apr 2023, one formalin-fixed and one ethanol-fixed crabs ( Hyastenus sp. ) with polyps on their carapaces, the latter specimen used exclusively for DNA extraction, GenBank: OR872049–OR872051 (16S), OR872005– OR872007 (18S), OR872029–OR872031 (28S) (the sequences correspond to 3 individual hydroid polyps); OR876279 is the COI sequence obtained from the Hyastenus crab.
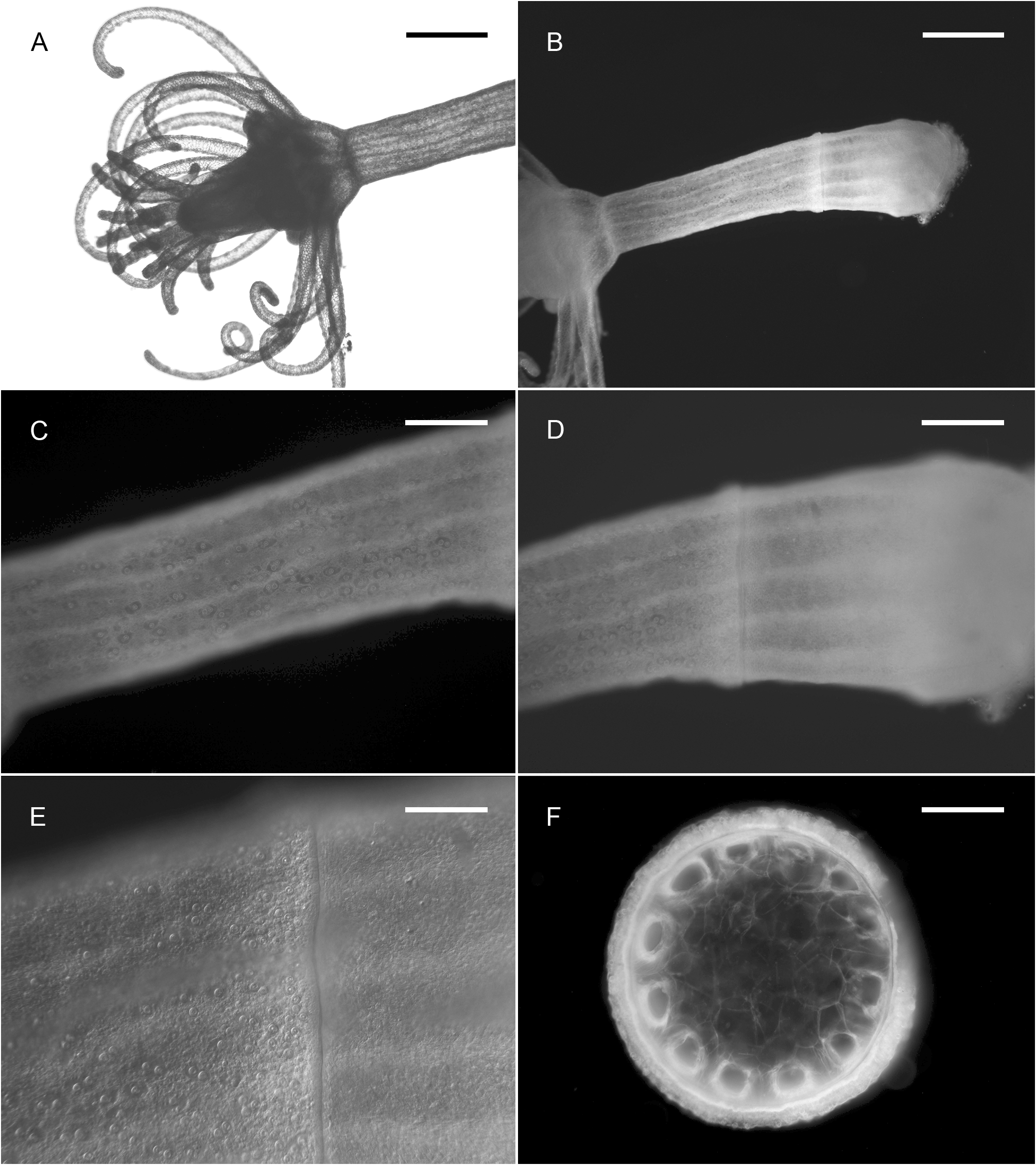
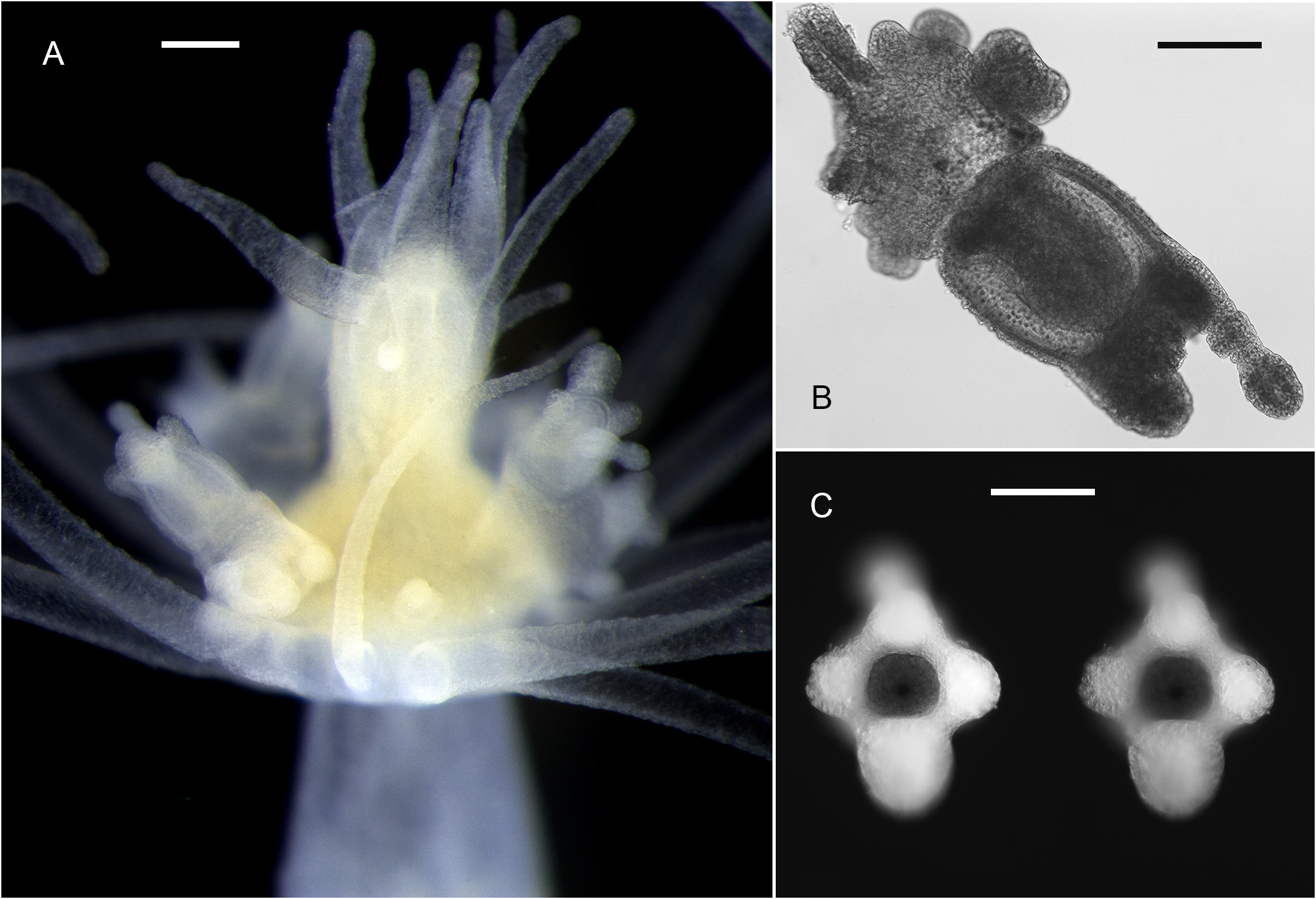
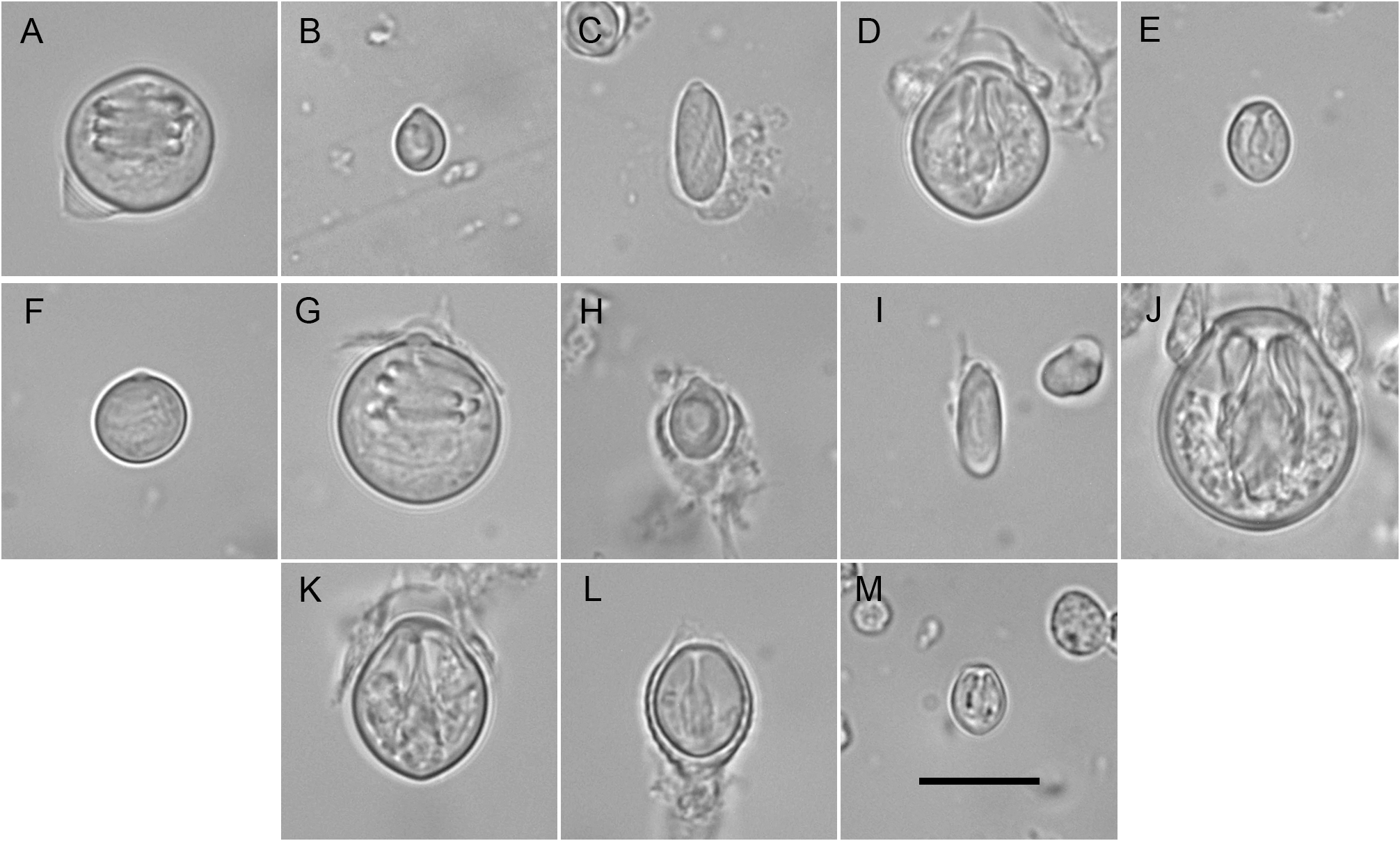
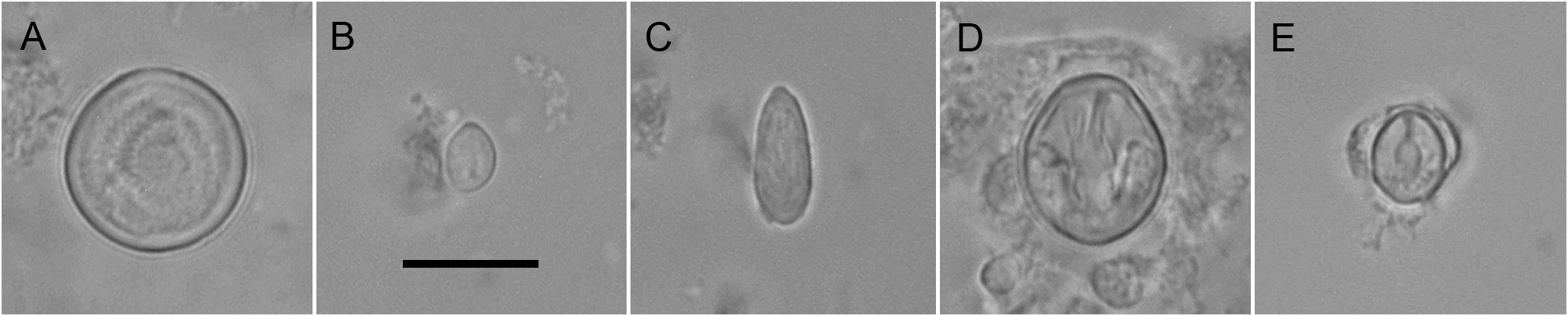
Description. Colonies invariably associated with a decapod host of the genus Hyastenus ( Fig. 1 View FIGURE 1 ); composed of numerous polyps fixed exclusively to the dorsal side of the carapace, not only restricted to the cephalothorax, but also to the eight walking legs; a stolonal web connecting the polyps to one another is obviously absent, and each of them is attached individually to the crab carapace by simple adhesion, as demonstrated by the agglutination of silt particles around the bases of hydrocauli; the latter, depending on the age of the polyps and their state of contraction, are 795–1790 µm long in the menthol-relaxed, formalin-fixed specimens examined herein; cauli ( Fig. 2 View FIGURE 2 ) gradually tapering distally, divided into two distinct parts ( Fig. 3B View FIGURE 3 ): a proximal one, usually accounting for about 1/3 of their total length, and the remaining 2/3 distal portion, both delimited by a transverse, 205–380 µm wide furrow ( Fig. 3D View FIGURE 3 ); a dozen peripheral, rather broad, unbranched, longitudinal canals (not forming lateral diverticuli) in the endoderm, spanning from one end to the other of the caulus ( Fig. 3F View FIGURE 3 ); core of the latter composed of large, polygonal, parenchyme cells, leaving centrally a narrow lumen ( Fig. 3F View FIGURE 3 ); proximal part of cauli covered by flimsy, highly transparent, closely appressed perisarc layer, epidermis not provided with nematocysts, and coenosarc not forming papillae; distal part of cauli naked, epidermis with scattered, ovoid, transparent patches comprising 1–2 large nematocysts in their center ( Fig. 3C View FIGURE 3 ); distally, a 175–310 µm wide constriction at junction between the caulus and hydranth base ( Fig. 3A, B View FIGURE 3 ). Hydranths flask-shaped ( Fig. 3A View FIGURE 3 ), 515–900 µm high and 440–675 µm wide; an internal, transverse septum divides them into a lower, relatively shallow, non-digestive part, filled with parenchyme cells, and an upper, comparatively taller, hollow, digestive part; a whorl of 10–15 aboral tentacles near their bases, and 8–11 oral tentacles, equally in a whorl, surrounding distally a dome-shaped hypostome ( Figs 2 View FIGURE 2 , 3A View FIGURE 3 , 4A View FIGURE 4 ); aboral tentacles 755–1990 µm long, gracefully arching upwards, slightly flattened laterally, gradually tapering distally, core solid, of pseudofiliform type; oral tentacles 230–440 µm long, basal 1/3 flattened laterally and adnate to the hypostome, distal 2/3 being free and arching outwards, with circular cross section, tapering only distally, surface rough due to the presence of irregular, vaguely annular clusters of nematocysts. Up to five short, conical blastostyles in a whorl, a short distance above the aboral tentacles ( Fig. 4A View FIGURE 4 ); each blastostyle giving rise to a reduced number of medusa buds at various stages of development ( Fig. 4B View FIGURE 4 ); buds before liberation ( Fig. 5B View FIGURE 5 ) with a 450–470 µm high and 360–400 µm wide umbrella, circular in cross-section; exumbrella provided with numerous, scattered, small, spherical nematocysts; mesoglea thin; manubrium club-shaped, extending almost to the closely-stretched velum, ending in pore-shaped mouth; four narrow radial canals ending basally in circular canal; four solid, perradial tentacle bulbs of inequal size: one bearing a club-shaped, 345–450 µm long, capitate tentacle, a conspicuouslyswollen, 305–325 µm long bulb opposite to it, as well as two comparatively less developed, 180–200 µm long, lateral bulbs; capitate tentacle with moderately-long stem ending distally in conspicuous, ovoid nematocyst knob, sometimes with an additional, intermediate, circular cluster, suggesting a moniliform structure in mature medusae; sense organs absent, and no gonads produced around the manubrium at this stage. Cnidome: its composition is summarized in Table 1 View TABLE 1 and the various capsules are illustrated in Fig. 6 View FIGURE 6 . Except for the unmistakable stenoteles and desmonemes, all the remaining capsules were seen undischarged, and their assignation to a given type was not attempted, until additional observations on living specimens are done.
Colors in life: basal part of caulus and endodermal canals milky-white, remainder of caulus, as well as the tentacles, translucent; hydranth base yellow-orange, hypostome white; manubrium and tentacle bulbs of the medusa white to yellow.
Remarks. Multi-locus and COI phylogenetic reconstructions revealed that Balinese C. balssi sequences are different from any other sequenced Corymorpha species ( Fig. 23A View FIGURE 23 ).
It is unclear how the hydroid colony is established on the carapace of its decapod host. Given that the dispersive stage is a free-swimming medusa produced in small numbers, the settlement of planula larvae seems statistically unlikely. No apparent stolonal web connects the polyps between them, and all individuals carried by a crab could not be regarded as a colonial animal stricto sensu. Some polyps show large, formless, basal bulges, suggesting that they may reproduce asexually by budding, thus forming clonal populations. It is possible that some polyps are either picked up by uncolonized crabs from individuals already bearing hydroids on their carapaces, or that crabs promote asexual spreading of the associated hydroid, as observed in the association between Lybia leptochelis crabs and Alicia sea anemones ( Schnytzer et al. 2017).
The short rooting filaments illustrated by Stechow in his unpublished plate [ Ruthensteiner et al. (2008: fig. 7B) and Fig. 5A View FIGURE 5 herein] are likely artefacts (as demonstrated by the examination of many polyps, including those mounted on the five microslides belonging to the type), nor could they be found in our specimens, in which the polyps simply adhere basally to the crab carapaces.
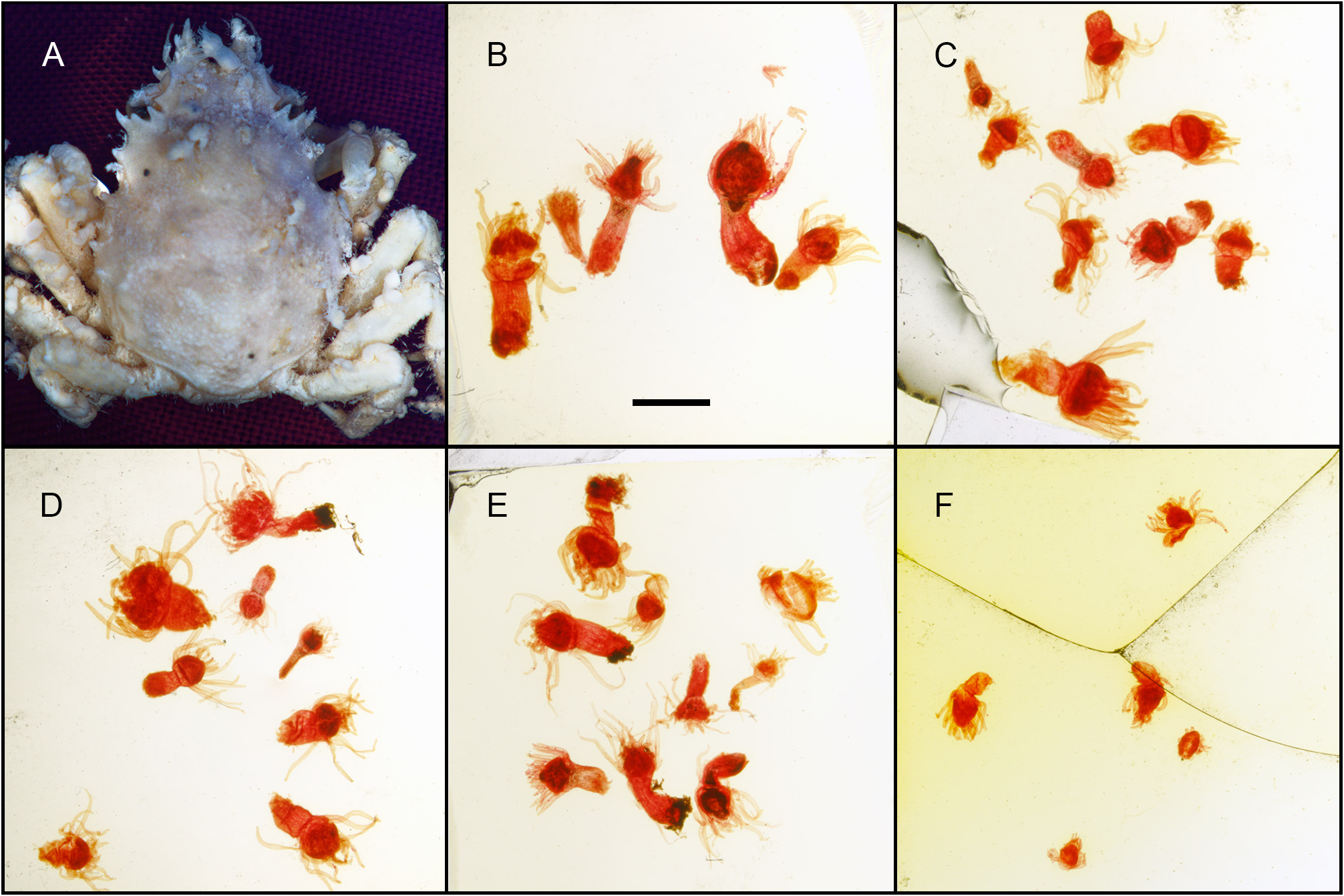
Syntype material, including alcohol-preserved specimens and microslide preparations ( Fig. 7 View FIGURE 7 ) were reexamined. The polyps 1 proved virtually indistinguishable from the new material at hand, except for their proportionally slightly bigger size (2–3.5 mm high, as reported in the original account). It should be stated that significant size variations were encountered in our specimens from Bali, depending on the age of the polyps and the size of their crab host; consequently, we consider the differences noted with the type to be intraspecific. The cnidome composition of the type specimens ( Fig. 8 View FIGURE 8 ) is the same as that of the material at hand (compare to Fig. 6A–E View FIGURE 6 ). Stechow (1932: 82) stated 2 that his colonies were mostly composed of infertile hydranths, and only one proved fertile and had very young, spherical gonophores, “apparently” provided with “one large and two lateral, poorly-developed tentacles” ( Fig. 5A View FIGURE 5 , enlargement); that polyp could not be located.
Although Stechow could not be certain in his observations, the medusa buds in our material possess a main, relatively long tentacle with a terminal capitation, a large tentacle bulb opposite to it, and a pair of globular tentacle bulbs laterally ( Fig. 5B View FIGURE 5 ). Some hydroid colonies photographed in the field show medusa buds with a relatively distinct, though small, intermediate ring of nematocysts, suggesting that the main tentacle in free-swimming, well-developed specimens is likely of moniliform type. This leads us question the identity of the mature medusa among the congeners described so far based on their planktonic stage. In an earlier paper, one of us ( Galea 2023: Appendix 1) provided a tabulated list of species of Corymorpha considered as valid. Among those occurring in the tropical Indo-Pacific, C. bitungensis (Xu, Huang & Guo, 2013) ( Fig. 5C View FIGURE 5 ), described from the Lembeh Strait, Indonesia, an area where the presence of the C. balssi is clearly documented in a series of underwater photographs available online (e.g. Levantovsky 2012), is a likely candidate. According to the original account, the medusa of C. bitungensis reaches up to 3 mm in bell height by 1.8 mm in width; its club-shaped manubrium projects for about 2/3 into the subumbrella; the main tentacle is provided with 3–10 intermediate rings of nematocysts besides the comparatively larger, terminal knob; the opposite tentacle bulb is quite well-developed and club-shaped, while the two laterals are similar, though shorter and slenderer ( Lin et al. 2013: 246, 248–250). While providing an account on their supposedly new species, no comparison was made by these authors with the previously-described C. juliephillipsi ( Gershwin, Zeidler & Davie, 2010) (see original account) from Moreton Bay, QLD, Australia, a nominal species with a very similar, if not identical, morphology and size ( Gershwin et al. 2010: 59, 62). The hydroid of C. balssi was essentially described based on material from Shark Bay, WA, Australia ( Stechow 1932), a locality that is located at nearly the same latitude as Moreton Bay, but on the opposite (western) coast of Australia, where no records of C. balssi from QLD came to our knowledge.
The colonies of C. balssi studied by Stechow (1932) reportedly occurred on Schizophrys dama (Herbst, 1804) (Crustacea: Decapoda : Majoidea: Majidae ). Indeed, one of his crabs [ Fig. 7A View FIGURE 7 , but see also Ruthensteiner et al. (2008: fig. 3E)] corresponds to the syntype illustrated by Lee et al. (2018: fig. 3). However, the Indonesian host is a yet to be identified, possibly new species of Hyastenus belonging to the H. inermis group (Crustacea: Decapoda : Majoidea: Epialtidae ) (P.K.L. Ng, pers. comm.), which is known to associate with cnidarians ( Lee & Ng 2019).
Stechow’s (1932: 82) material was collected from Shark Bay in June, August and September 1905, and from Onslow (> 500 km to the N) in July of the same year. According to SeaTemperature.org, the average temperatures for Shark Bay are 20.97°C (18.83‒23.72°C) in June, 20.03°C (18.51‒22.72°C) in August, and 20.50°C (18.98‒ 22.57°C) in September, and for Onslow 22.59°C (20.51‒25.26°C) in July. Conversely, the annual minimum temperature recorded for Bali is 26.8 °C (25.3‒28.4°C) in September, but the temperature rises to as much as 29° C (27.9‒30.1°C) in April, when our material was collected, indicating that C. balssi can live under different ecological conditions.
Distribution. Australia: Shark Bay and Onslow, WA ( Stechow 1932); Indonesia: Bali (present study), Borneo, Sumbawa, Komodo Archipelago, Lembeh Strait, Ambon (based on photographs available on Flickr, iNaturalist, Instagram and Facebook).
| ZSM |
Bavarian State Collection of Zoology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Hydroidolina |
|
Order |
|
|
Family |
|
|
Genus |
Corymorpha balssi Stechow, 1932
| Galea, Horia R. & Maggioni, Davide 2024 |
Euphysora juliephillipsi
| Gershwin, L. A. & Zeidler, W. & Davie, P. J. F. 2010: 59 |
Corymorpha (Euphysa) balssi
| Ruthensteiner, B. & Gotz-Nodo, R. & Straube, N. 2008: 13 |
| Watson, J. E. 2008: 187 |
| Stechow, E. 1932: 82 |