Galearctus avulsus, Yang & Chen & Chan, 2011
|
publication ID |
https://doi.org/ 10.5252/z2011n2a4 |
|
persistent identifier |
https://treatment.plazi.org/id/03A387AD-FFC6-522F-FCE8-FD5F4D98F703 |
|
treatment provided by |
Felipe |
|
scientific name |
Galearctus avulsus |
| status |
sp. nov. |
Galearctus avulsus n. sp.
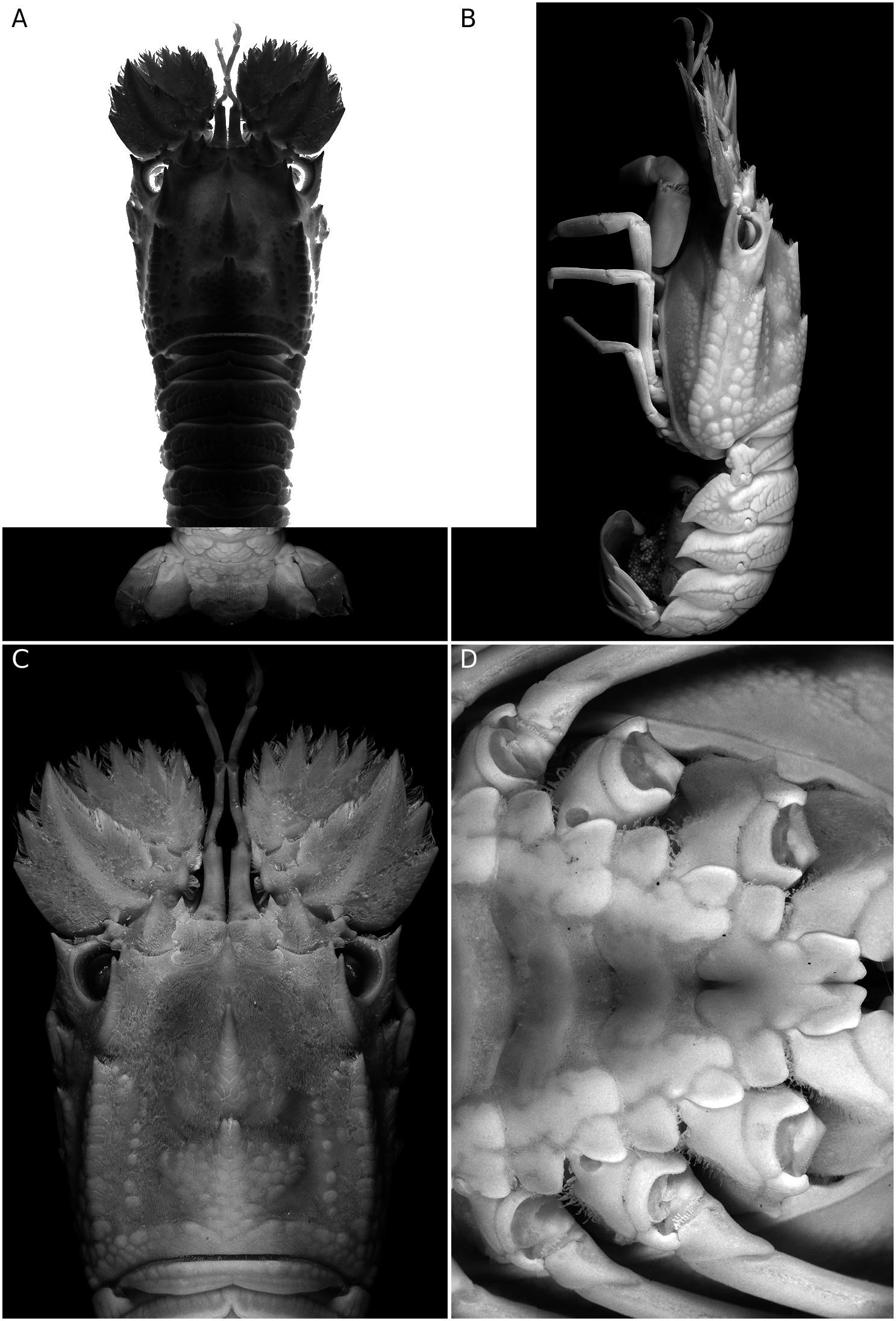
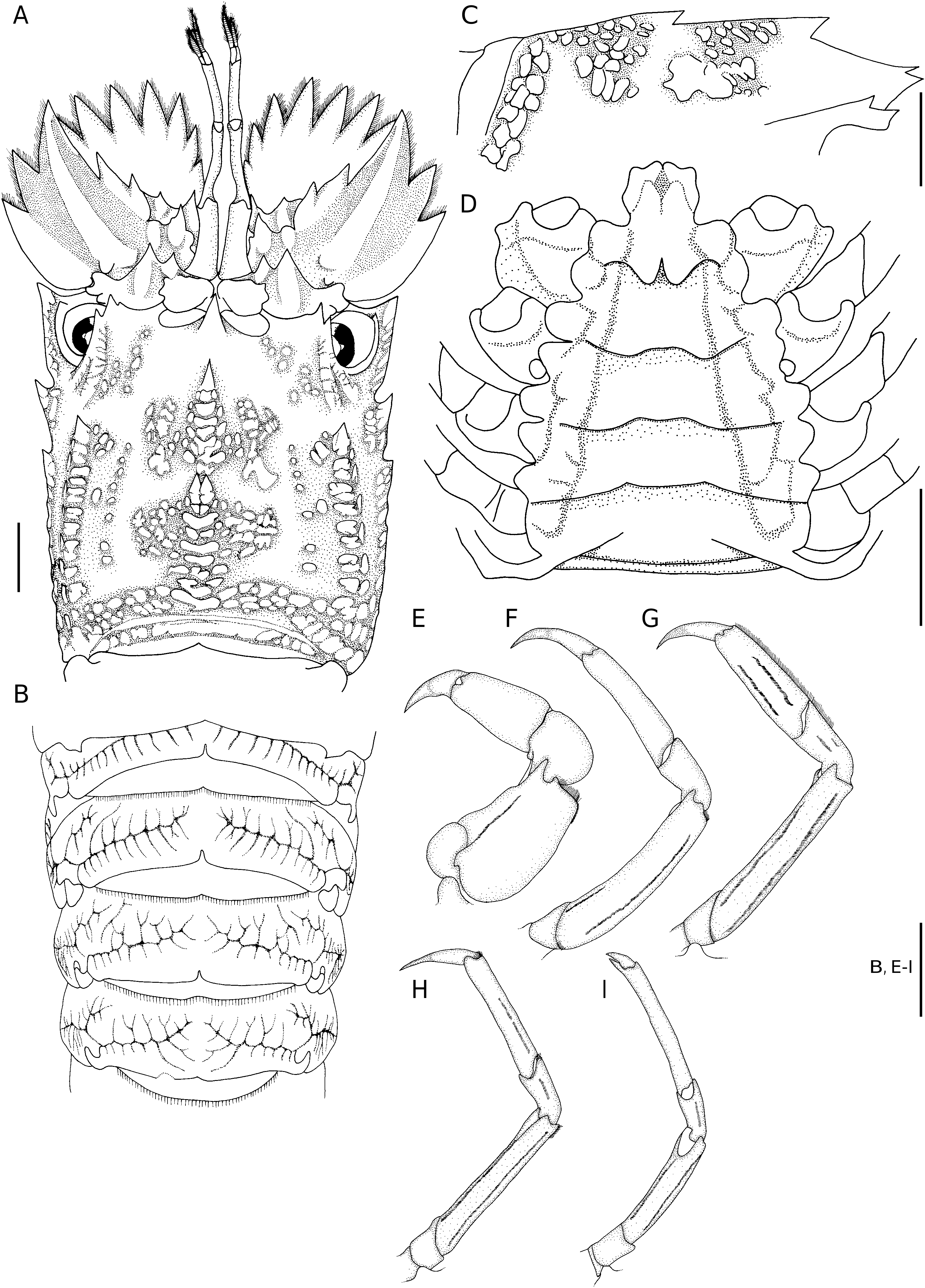
( Figs 1 View FIG ; 2 View FIG )
Galearctus kitanoviriosus View in CoL – Holthuis 2002: 565 View Cited Treatment (in part), fig. 25 (not Harada 1962).
TYPE MATERIAL. — Holotype: New Caledonia. BERYX 11 , stn CP 22, 24°44’S, 168°07’E, 490-510 m, 17.X.1992, 1 ovig. ♀, 31.0 mm cl (MNHN-IU-2010-1910). GoogleMaps
Paratypes: New Caledonia. Norfolk Ridge, SMIB 3, stn DW 14, 23°40’S, 168°00’E, 246 m, 22. V.1987, 1 ♀, 26.0 mm cl (MNHN-Pa 1274). — Matthew Island, VOLSMAR, stn DW 39, 22̊20.5 ’S, 168̊43.5 ’E, 305 m, 8. VI.1989, 1 ♀, 26.0 mm cl (MNHN-Pa 1384). — BERYX 11, stn CH 26, 24°42’S, 168°08’E, 230-260 m, 17.X.1992, 1 ovig. ♀, 26.0 mm cl (MNHN- IU-2010-1911). — BATHUS 1, stn CP 711, 21̊43.16 ’S, 166̊36.35 ’E, 315-327 m, 13. V.1993, 1 juv., 9.0 mm cl (MNHN-Pa 1859). — HALIPRO 1, stn CP 851, 21̊43 ’S, 166̊37 ’E, 314-364 m, 19. V.1994, 1 ơ, 11.0 mm cl (MNHN-Pa 1861). — HALICAL 1, stn 2, 20°47.5’S, 164°52’E, 658-680 m, 23.XI.1994, 1 ơ, 13.0 mm cl (MNHN-Pa 1860).
Chesterfield Islands. Dredge, 250 m, 22. V.1979, 1 ♀, 26.8 mm cl (MNHN-IU-2009-46).
TYPE LOCALITY. — New Caledonia, 24°44’S, 168°07’E, at depths of 490- 510 m.
ETYMOLOGY. ― The Latin avulses, alluding to the meaning which is tear away or avulsion, refers to the longitudinal median groove at the posterior part of the thoracic sternite I being distinctly widen posteriorly and somewhat like torn away in the new species. All other known species of Galearctus have very narrow median longitudinal groove at the posterior part of the thoracic sternite I.
DISTRIBUTION. ― South Pacific from New Caledonia, Chesterfield Islands, and probably Fiji, at depths of 230- 680 m.
COLORATION. — Not known.
DESCRIPTION
Carapace ( Figs 1 View FIG A-C; 2A, C) with squamiform tubercles and short pubescence. Rostral tooth well developed, sharply pointed and hanging over base of antennular somite. Pregastric tooth completely absent.Gastric tooth moderately elevated and about 1.5 times as high as rostral tooth, followed posteriorly by 6 transverse rows of squamae, each row with 3 or 4 fused squamae.Anterior submedian ridge divided into two lines; inner line composed of 6 or 7 larger but irregular squamae; outer line with smaller and fewer squamae. 2 or 3 tubercles present between postrostral and anterior submedian ridges. Cardiac tooth deeply bifurcate and distinctly elevated, followed posteriorly by 6 or 7 transverse rows of 1-3 large flattened squamae. Posterior postrostral ridge flanked with patch of 17 or 18 squamae, making posterior submedian ridges indistinct. 7 or 8 distinct intermediate tubercles present. 1 large squama present between intermediate tubercles and posterior branchial ridge. Cervical groove shallow and relatively narrow, thus anterior and posterior branchial carinae relatively close. Anterior branchial ridge bearing 2 large, sharp teeth anteriorly. Posterior branchial ridge with anterior end forming an acute tooth, followed posteriorly by double row of 8 or 9 squamiform tubercles. Postorbital ridge not sharp. Intercervical ridge composed of 4 or 5 squamae. Anterolateral, mediolateral and posterolateral teeth as flattened squamiform tubercles except anteriormost tubercle of each part which forms a strong tooth, posterolateral part with 10 or 11 squamae. Posterior marginal groove deep, narrow. Posterior margin of carapace medially incised.
Abdomen ( Figs 1A, B View FIG ; 2B View FIG ) with arborescent sculpture, median areas only slightly elevated and without dorsal carina. Articulate surfaces of each tergite more or less smooth, only tergite II always with shallow interrupted transverse grooves.Posterior margins of tergites I to III deeply incised medially, that of IV weakly incised medially. Pleura II to IV sharply pointed posteroventrally, posterior margins smooth ( Fig. 1B View FIG ). Posterior margin of telson bearing 2 pairs of teeth (outer pair larger) and without spines in-between.
Anterior margin of antennal segment VI with 7 teeth, outer six large and slender while inner one obtuse and short. Antennal segment IV bearing 2 distinct teeth on anterior margin; outer margin armed with 2 large teeth; dorsal surface with only 1 oblique median carina. Fused antennal segments II and III with 2 teeth on anterior margin, outer tooth distinctly larger than inner tooth ( Figs 1C View FIG ; 2A View FIG ).
Pereiopod I stout and shorter than other pereiopods ( Fig. 2E View FIG ). Propodus of pereiopod II slender; about 1.5 times as long as dactylus ( Fig. 2F View FIG ). Propodus of pereiopod III not subchelate, distinctly wider and flatter than that of pereiopod II, bearing 2 long and parallel hairy longitudinal grooves on outer surface ( Fig. 2G View FIG ). Pereiopod IV sometimes with 1 interrupted hairy groove on outer surface of propodus ( Fig. 2H View FIG ). Carpi of pereiopod III to V each with 0 or 1 hairy groove on outer surface of carpus and 1 or 2 hairy grooves on outer surface of merus ( Fig. 2 View FIG G-I).
Anterior margin of thoracic sternum produced forwards, tongue-like and with anterolateral angles distinctly produced; median part of anterior margin of tongue-like process triangularly protruded but medially incised, incision continuous posteriorly as a groove that is sometimes triangularly expanded medially ( Figs 1D View FIG ; 2D View FIG ). Entire median part of thoracic sternum deeply sunken, only lateral borders bluntly elevated. Well-developed and rounded transverse carinae separating each thoracic sternite. Only short and posteriorly divergent median longitudinal groove present at posterior part of sternite I (base of pereiopod I). Last thoracic sternite sometimes bearing median tubercle, posterolateral angle unarmed in females but developed into small tooth in males.
Eggs small and numerous, about 0.5 mm in diameter.
REMARKS
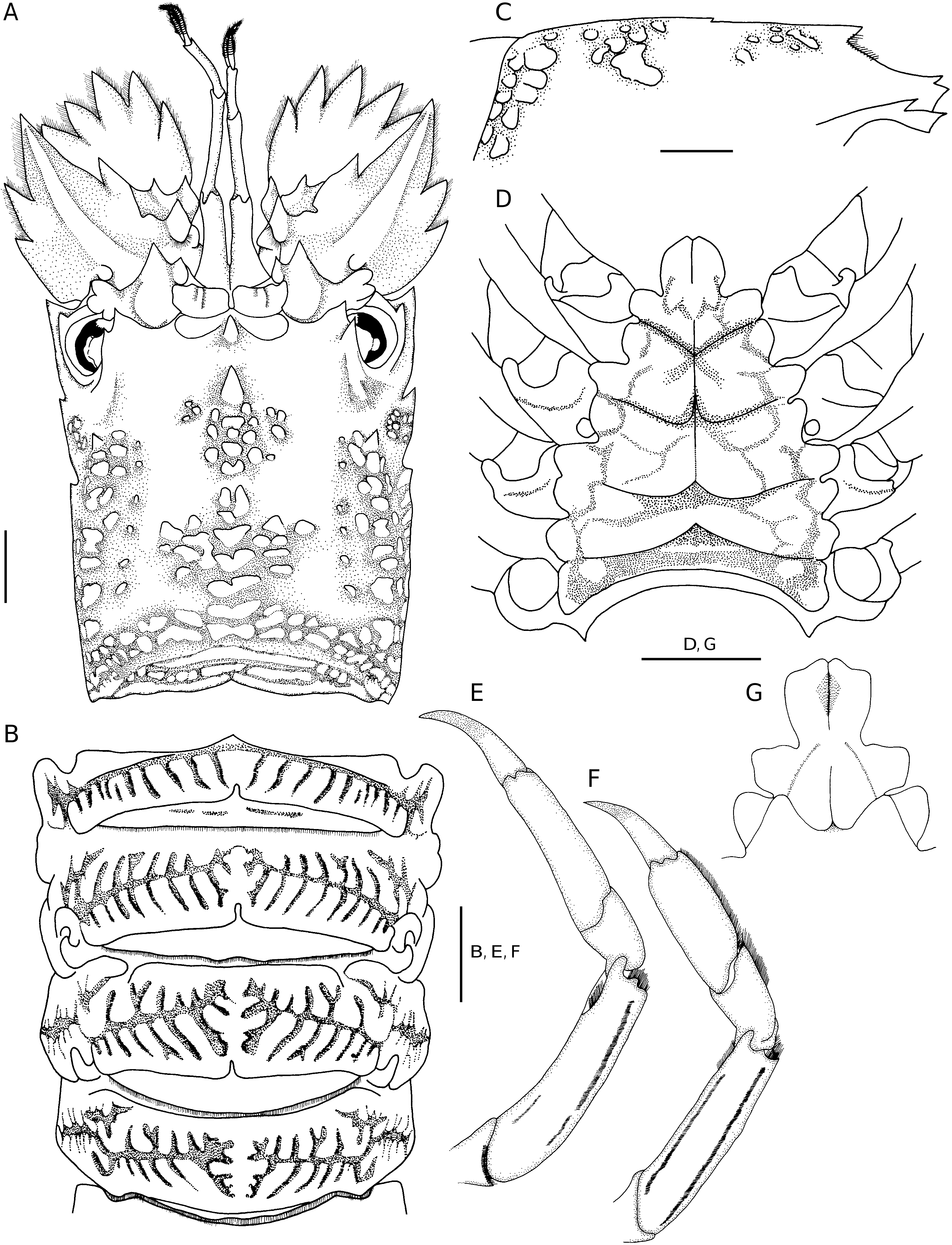
The present new species is separated from G. kitanoviriosus by the following characters: 1) anterior margin of antennal segment IV with 2 distinct teeth in G. avulsus n. sp. ( Figs 1A, C View FIG ; 2A View FIG ), but only with one tooth in G. kitanoviriosus ( Fig. 3A View FIG ); 2) cardiac tooth distinctly elevated ( Figs 1B View FIG ; 2C View FIG ) in G. avulsus n. sp., but very low and almost level with dorsal carapace in G. kitanoviriosus ( Fig. 3C View FIG ); 3) both anterior and posterior submedian ridges composed of many more squamae in G. avulsus n. sp. ( Figs 1A, C View FIG ; 2A View FIG ) than in G. kitanoviriosus ( Fig. 3A View FIG ); 4) outer surface of propodus of pereiopod III always with two long setal grooves in G. avulsus n. sp. ( Fig. 2G View FIG ), but usually without grooves (70%) or rarely (30%) with 2 short setal grooves in G. kitanoviriosus ( Fig. 3F View FIG ); 5) anterior extension of the thoracic sternum tongue-like, and with its anterolateral angles distinctly protruded in G. avulsus n. sp. ( Figs 1D View FIG ; 2D View FIG ), but rather triangular and with its anterolateral margins oblique in G. kitanoviriosus ( Fig. 3D View FIG ); and 6) G. avulsus n. sp. has the thoracic sternum, including the posterior half of sternite I, distinctly sunken medially, a medial longitudinal groove is only present on the posterior half of sternite I, and each sternite is separated by distinct transverse ridges ( Figs 1D View FIG ; 2D View FIG ). In G. kitanoviriosus , the thoracic sternum is only weakly depressed medially, but bears a distinct median longitudinal groove along the anterior half of the sternum, and each sternite is separated by transverse grooves and not by ridges ( Fig. 3D View FIG ).
Besides the differences listed above, the posterolateral angles of the last thoracic sternite are sometimes developed into teeth in G. kitanoviriosus females ( Fig. 3D View FIG ), but always unarmed in G. avulsus n. sp. females ( Fig. 2D View FIG ). Moreover, the articulate surfaces of the abdominal tergites II to IV sometimes also bear shallow interrupted transverse grooves in G. kitanoviriosus ( Fig. 3B View FIG ), but these areas are always smooth in G. avulsus n. sp. ( Fig. 2B View FIG ). Galearctus avulsus n. sp. appears to be restricted to waters deeper than 200 m, while G. kitanoviriosus is distributed in less than 100 m deep. For the New Caledonia and Chesterfield Islands material assigned by Holthuis (2002) to “ G. kitanoviriosus ” and deposited at MNHN, all but one specimen are actually G. avulsus n. sp. and they were all collected from 230-680 m deep. Only the specimen registered as MNHN-Pa 775 is truly G. kitanoviriosus , and it is the only one collected from shallow water (8-15 m). Another New Caledonian specimen from the University of Florida (UF 1022) was also available for the present study. This specimen was collected from 0.5-1.5 m deep, and has both the morphological characters and typical coloration of G. kitanoviriosus . The live coloration of G. avulsus n. sp. is so far unknown. Holthuis (2002) mentioned that there are three specimens from Fiji deposited in the MNHN that belong to “ G. kitanoviriosus ”, however they can not be located at present. As these three Fiji specimens were collected from 316-357 m deep, they most likely belong to G. avulsus n. sp. Moreover, there are two more specimens mentioned by Holthuis (2002) from New Caledonia and deposited in the National Museum of Natural History, Washington, D.C. (USNM 1000666), and Rijksmuseum van Natuurlijke Historie, Leiden (RMNH D 48767). As they were also collected from deeper water (247-290 m), they are also likely to belong to G. avulsus n. sp.
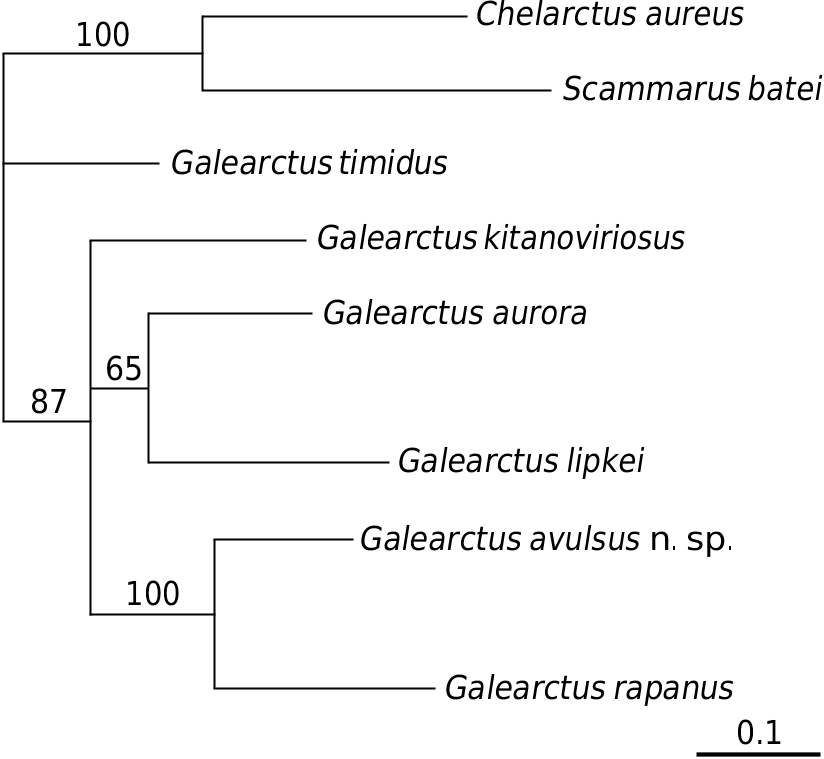
A comparison amongst the 657 bps of the barcoding mitochondrial COI gene ( Table 2) shows that there is a 15.2% sequence divergence between G. avulsus n. sp. and G. kitanoviriosus, which is higher than that between G. aurora and G. lipkei (13.8%). A difference of 15.2% divergence in the partial sequence of COI is generally considered to be more than sufficient to separate species in decapod crustaceans ( Daniels et al. 2002; Ravago & Juinio-Meñez 2002; Cabezas et al. 2009). The available partial COI gene sequences for the Scyllaridae show the interspecific divergence of species in this family ranging from 2 to 25% ( Burton & Davie 2007; Yang et al. 2008; Palero et al. 2009; Yang & Chan 2010). On the other hand, the intraspecific sequence divergence of G. kitanoviriosus is only 0.4% between the Taiwanese and New Caledonian material (unfortunately attempts to obtain the sequence for the Japanese material failed). The genetic data ( Fig. 4 View FIG ) suggest that the present new species is actually most similar to G. rapanus (14.5%). Indeed, G. avulsus n. sp. and G. rapanus (4 specimens examined: Australes Islands, 2 ♀♀ 27.7-30.7 mm cl, NTOU M00865 View Materials ; Lord Howe Island, 2 ♀♀ 19.5-24.3 mm cl, QM W29060) are morphologically quite similar and with small specimens almost identical with others. Although large specimens of G. rapanus can be readily separated from G. avulsus n. sp. by having the outer tooth of the antennal segments III extraordinarily large and the thoracic sternum distinctly narrower, these differences are not so obvious between smaller individuals. This is partly due to the outer surface of the propodus of the pereiopod III in G. rapanus that may bear 1 or 2 setal grooves (1 setal groove in the Australes Islands, 27.7 mm cl female and Lord Howe Island 19.5 mm cl female, 2 setal grooves in the Lord How Island 24.3 mm cl female) instead of always naked (Australes Islands 30.7 mm cl female), as described by Holthuis (1993, 2002). Nevertheless, the longitudinal median groove at the posterior part of the thoracic sternite I is distinctly widen posteriorly in G. avulsus n. sp. ( Figs 1D View FIG ; 2D View FIG ) but always very narrow and not posteriorly divergent in G. rapanus ( Fig. 3G View FIG ). Moreover, the posteriorly divergent median longitudinal groove at the posterior part of the thoracic sternite I in G. avulsus n. sp. is unique in the genus. All other known species of Galearctus have very narrow median longitudinal groove on the thoracic sternite I. The following revised key is given for the separation of the species in Galearctus .
| V |
Royal British Columbia Museum - Herbarium |
| VI |
Mykotektet, National Veterinary Institute |
| CH |
Circulo Herpetologico de Panama |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Galearctus avulsus
| Yang, Chien-Hui, Chen, Shiung & Chan, Tin-Yam 2011 |