Buffonellaria, CANU & BASSLER, 1927
|
publication ID |
https://doi.org/10.1111/j.1096-3642.2007.00379.x |
|
publication LSID |
lsid:zoobank.org:pub:D81F148F-7BA0-4E5A-9AE1-21A492ED5A78 |
|
persistent identifier |
https://treatment.plazi.org/id/03A3116F-2B50-3123-AA78-FB133E62FCB9 |
|
treatment provided by |
Felipe |
|
scientific name |
Buffonellaria |
| status |
|
GENUS BUFFONELLARIA CANU & BASSLER, 1927 View in CoL
Original description: Buffonellaria Canu & Bassler, 1927: 8 .
Synonyms: Buffonellaria: Hayward & Ryland, 1979: 204 ; Gordon, 1984: 117; Zabala & Maluquer, 1988: 127; Hayward & Ryland, 1999: 356.
Type species: Hippothoa divergens Smitt, 1873 .
Revised diagnosis: Colony encrusting, unilaminar to plurilaminar, or erect rigid bilaminar. Basal pore chambers present, vertical zooecium walls with multiporous septula. Frontal wall with a single row of marginal pores, central shield usually imperforate in adult zooids but occasionally with tiny pseudopores, a distinct medioproximal ooecial pore present in some zooids, extreme frontal hypercalcification masking primary frontal characteristics may occur. Primary orifice with sinus and condyles. No spines except on ancestrula and first generation autozooids. Ooecium of the ovicell recumbent on distal zooid’s frontal wall, prominent first, later usually immersed by secondary calcification, emerging from the margin of the ooecial pore situated in proximal frontal wall of distal zooid, gymnocystal ectooecium with large noncalcified central window, exposed frontal area of entooecium imperforate, with more or less prominent radiating ribs that partly cover the intervening coelomic lumen between ento- and ectooecium; not closed by zooidal operculum. Avicularia adventitious, generally adjacent to orifice; additional enlarged dimorphic avicularia absent or present, occasionally frequent on frontal, with acute mandible. Ancestrula with welldeveloped proximal gymnocyst and a distally located opesia surrounded by spines.
Remarks: The diagnosis differs in several aspects from the latest account by Hayward & Ryland (1999), which is mainly due to the fact that these authors used the same diagnosis as in their earlier description of the British Fauna in 1979. In the latter work B. armata ( Hincks, 1862) was included in the genus, which is, in contrast to Buffonellaria , characterized by the presence of oral spines, spatulate avicularia and a beaded orifice rim, and which is now placed in the genus Stephanollona Duvergier, 1921 .
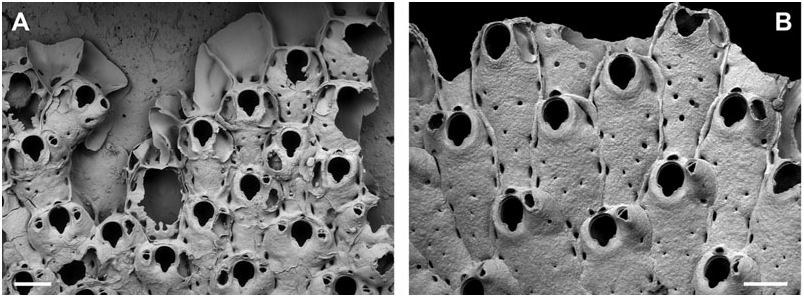
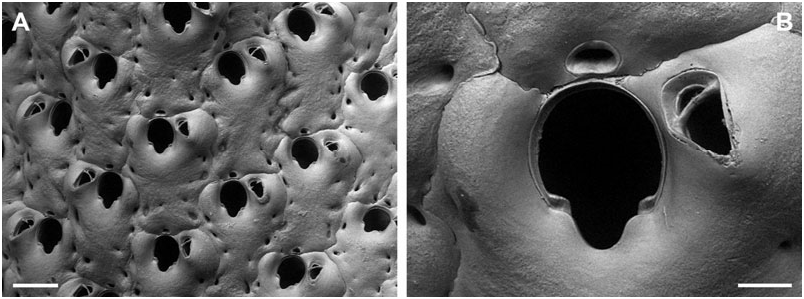
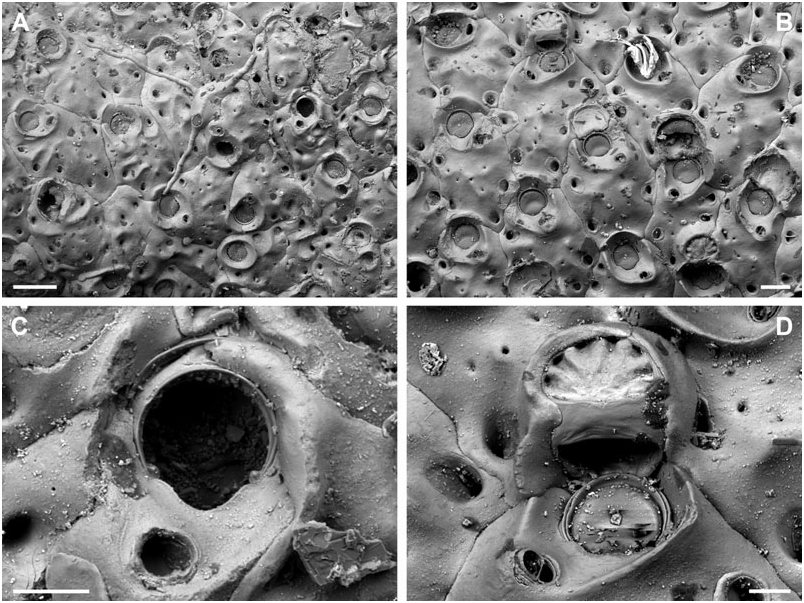
Furthermore, as for the Celleporidae , the genus Buffonellaria was hitherto considered to include species with an imperforate frontal wall only. SEM observations of the colony growth margin revealed, however, that in some species frontal pseudopores may exist during early ontogeny ( Fig. 1 View Figure 1 ), which soon become either completely closed, or remain open as frontal pseudopores of variable but generally very small size, depending on the taxon. A completely porous frontal shield may therefore be readily produced by paedomorphosis, i.e. when the early ontogenetic stage is retained in adult zooids. A clear line between species with and without a porous frontal wall (cf. Pourtalesella ), may therefore be difficult to draw. In fact, the presence of a pseudoporous frontal wall is the only significant feature distinguishing the very recently erected genus Pourtalesella ( type species Schizopodrella incrassata Canu & Bassler, 1928 ) from Buffonellaria . Orifice morphology and ovicell formation are identical in Pourtalesella , although Winston (2005: 95) mentions a ‘marginally perforated entozooidal [ sic] area’ in the ooecium, but this is a mistake that has occasionally been made (see below). The larger, dimorphic, frontal avicularium typical for most species of Buffonellaria is absent in P. incrassata , in which only monomorphic oral avicularia are additionally budded during later ontogeny. However, the larger frontal avicularia are also lacking in B. porcellanum Arístegui Ruiz, 1987 but avicularia would, in any case, not constitute a character justifying the distinction of genera (D.P. Gordon, pers. comm. 2006). More information on frontal wall formation and functional morphology in both Pourtalesella and Buffonellaria species is clearly needed to clarify the status of Pourtalesella .
In Buffonellaria and Pourtalesella (and possibly also in Torquatella ) ooecium production is decoupled from the formation of maternal zooids, which are characterized by having a distinct, rimmed, round or oval ooecial pore immediately distal to the orifice, deriving from the proximal frontal wall of the succeeding autozooid ( Fig. 2 View Figure 2 ). From this ooecial pore the ooecium may later, after complete formation of the frontal wall of the succeeding zooid, be generated. Therefore, ovicell production does not take place at the extreme colony periphery and can potentially be delayed until, e.g. environmental conditions have become favourable, while then being able to proceed at a greater rate without the colony having to produce maternal zooids at that stage. The ooecium is formed by evagination of the inner epithelial lining of the ooecial pore, initially producing a flat cuticular fold with subsequent calcification of ento- and ectooecium. An intervening coelomic lumen is present between these calcified layers. Calcification of the ectooecium commences to proceed frontally at a certain point, leaving a central semicircular area of exposed entooecium (the tabula). The generally semicircular area of frontal entooecium is superficially densely perforated by minute pits (±0.5 Mm), and usually bears radiating ribs. The entooecium is, nevertheless, imperforate, whereas it is the disappearing of the ribs and intervening grooves underneath the cuticular part of the ectooecium that wrongly gives the appearance of a row of pores along the exposed entooecial margin in bleached specimens. In several species frontal avicularia are budded and secondary calcification proceeds to grow immediately after completion of the ovicell – presumably for protective reasons because extreme types of hypercalcification are only observed in those colony parts where ovicells exist – thereby dramatically altering the zoarial surface morphology in this part.
An ancestrula was not observed in the present material due to overgrowth by later zooids during colony growth. According to Hayward & Ryland (1999) it has a well-developed gymnocyst and a distal semiorbicular opesia surrounded by a number of spines.
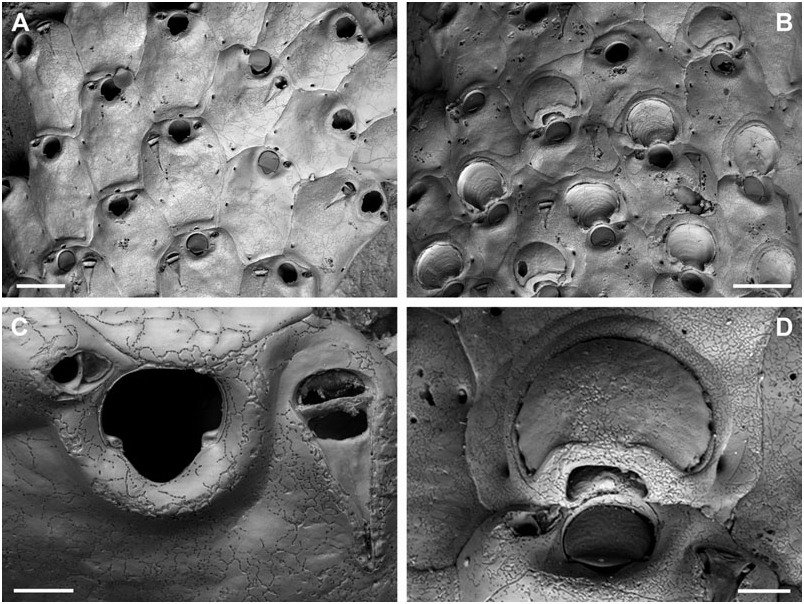
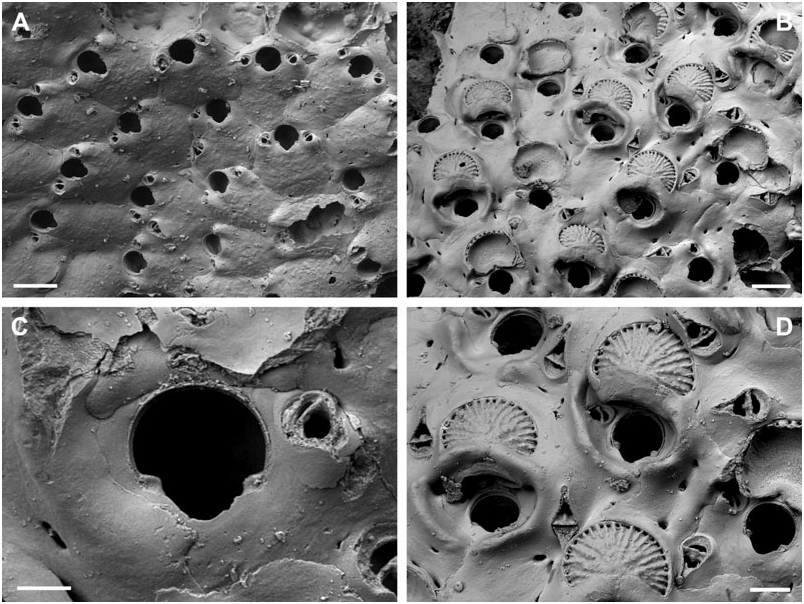
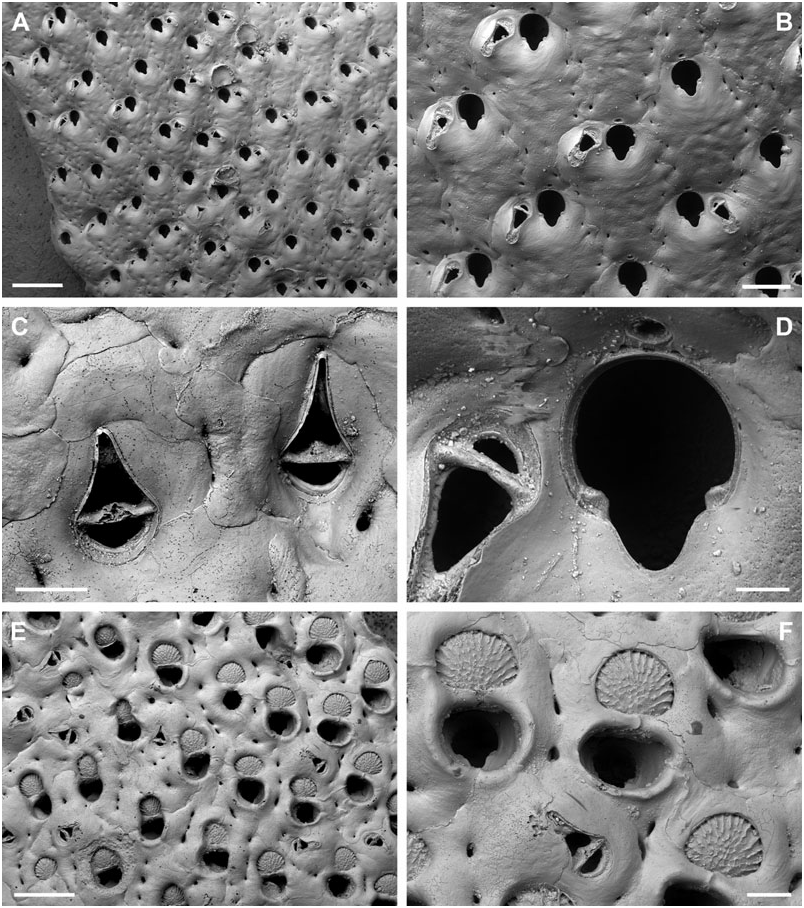
BUFFONELLARIA DIVERGENS ( SMITT, 1873) View in CoL
( FIG. 3A, B View Figure 3 )
Original description: Hippothoa divergens forma typica Smitt, 1873: 47, pl. 9, fig. 179.
Synonyms: non Buffonellaria divergens ( Smitt, 1873) : Canu & Bassler, 1928: 88, pl. 8, figs 7, 8.
Material examined: Lectotype (designated here): SMNH 1880 View Materials , Smitt Collection, leg. L.F. Pourtales 21.04.1869, Florida, off Key West , 250 m, on pebble; figured in Smitt (1873: pl. 9, fig. 179).
Measurements: ZL, 679 ± 70, 530–773 (1, 13); ZW, 526 ± 50, 413–587 (1, 13); OL, 134 ± 4, 129–140 (1, 10); OW, 127 ± 6, 117–136 (1, 10); oAL, 47 ± 5, 39–55 (1, 11); oAW, 35 ± 4, 28–42 (1, 11); fAL, 109 ± 6, 102– 113 (1, 3); fAW, 50 ± 3, 47–53 (1, 3).
Description: Colony encrusting, unilaminar, multiserial. Zooecia: hexagonal to polygonal, separated by shallow grooves. Frontal wall slightly convex, wrinkled, imperforate except for between two and five round or oval marginal pores. Primary orifice about as long as wide, surrounded by a very slightly raised but broad rim; anter broadly horseshoe-shaped, proximal margins short and straight with rounded shoulders, passing into a relatively narrow and widely U-shaped sinus, occupying about two-thirds of the total width.
Ovicells were not present in the available material.
Oral avicularia: usually paired, oval, lateral to orifice at about mid-distance, situated on a raised cystid with one or two small basal pores, frontal plane at an acute angle to colony surface; rostrum semi-elliptical, directing proximolaterally. Additional frontal avicularia common, even on zooids at the colony periphery, situated at the lateral zooid margin just distal to mid-distance of zooid, on a very slightly raised cystid with two small areolar pores at the base, rostrum elongate triangular, parallel-sided and slightly downcurved distally, pointing laterally, at an acute angle to colony surface.
Remarks: When introducing the new genus Buffonellaria, Canu & Bassler (1927) chose Hippothoa divergens Smitt, 1873 as the type species. However, a lectotype of B. divergens did not exist, and there were no specimens of this species in the bryozoan collection at the MCZ, which formed the basis of Smitt’s (1873) publication on ‘Floridan Bryozoa’, and which was recently redescribed by Winston (2005). The remaining material from Florida not stored at the MCZ, is stored in the Swedish Natural History Museum, and we here designate the lectotype of B. divergens (SMNH 1880) . The type specimen is the colony shown by Smitt (1873: pl. 9, fig. 179), as is evident from the location of the three frontal avicularia shown in Fig. 3A View Figure 3 . Unfortunately, this specimen lacks ovicells, and bleaching of the type in order to observe orifice characteristics was, understandably, not allowed. The redescription is therefore restricted to superficial zooecial and avicularian morphology only.
As Winston (2005) rightly remarked, the B. divergens specimens from Florida differ from the eastern- Atlantic and Mediterranean specimens that are also referred to this species; this will be demonstrated in detail below. Consequently, B. divergens is not, as was hitherto assumed, a widely distributed, trans- Atlantic species, but is restricted to Floridan and, possibly, Caribbean waters. However, as Winston noted, even the specimens collected in the area of the Tortugas during a later Blake cruise, which were identified as B. divergens , differ significantly in zooecium size from the lectotype. Therefore, in light of the present results that show relatively restricted geographical species ranges, other records of this species even from nearby regions need to be considered cautiously.
Canu & Bassler’s (1928: 88, pl. 8, figs 7, 8) B. divergens from Cuba is a distinct species because of the lack of frontal avicularia and the distally directing oral avicularia. Furthermore, as they do not mention or show the typical ooecium, it is questionable whether this species belongs to Buffonellaria at all. The same applies to another species from the Gulf of Mexico they newly describe as Buffonellaria reticulata Canu & Bassler (1928: 89 , pl. 8, fig. 5).
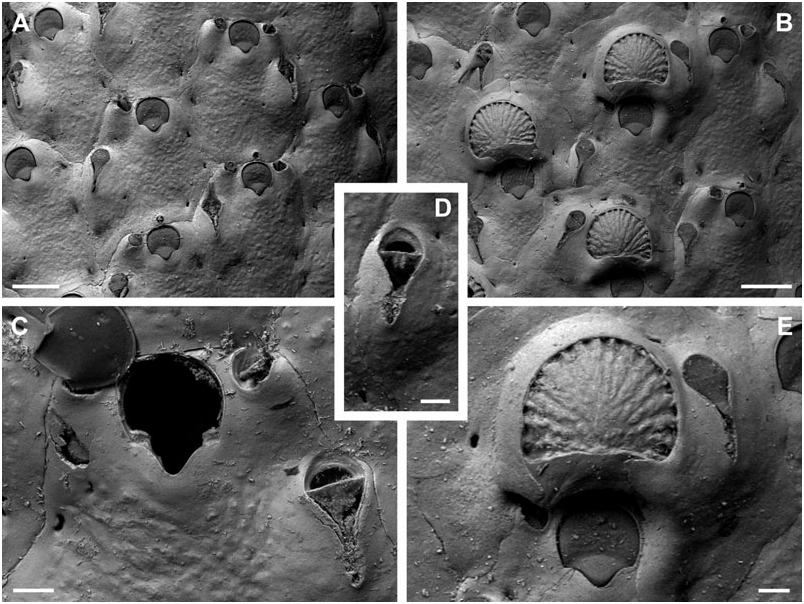
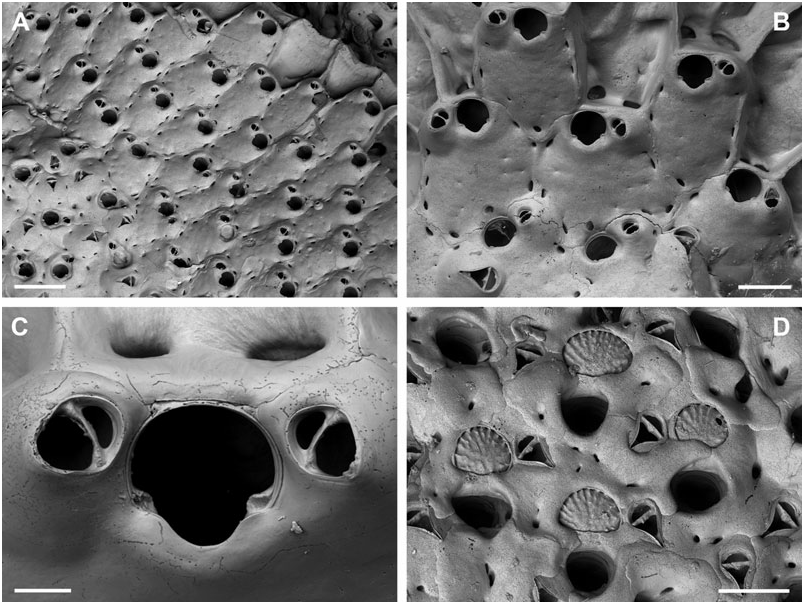
BUFFONELLARIA RITAE SP. NOV.
( FIG. 4A–D View Figure 4 )
Material examined: Holotype: NHM 2007.5.18.6, off Santa Cruz, Madeira, ‘Blake’ Cruise, Stn 182, 210 m, on Miniacina miniacea Pallas, 1766 .
Paratype: NHM 2007.5.18.7, off Santa Cruz, Madeira, ‘Blake’ Cruise, Stn 182, 210 m, occurring free of a substrate.
Other material: NHM 1911.10.1.1077, off Santa Cruz, Madeira, ‘Blake’ Cruise, Stn 182, 210 m; NHM 1911.10.1.1078, off Santa Cruz, Madeira, ‘Blake’ Cruise, Stn 182, 210 m, encrusting M. miniacea .
Differential diagnosis: B. ritae sp. nov. differs from all other species in the common presence of a dimorphic, proximally directing avicularium that develops during early ontogeny, and which is situated lateral to the orifice, as well as in its large and smooth exposed area of entooecium.
Etymology: Named in honour of the senior author’s mother, Rita Berning.
Measurements: ZL, 457 ± 50, 351–521 (3, 20); ZW, 353 ± 44, 248–427 (3, 20); OL, 97 ± 7, 79–109 (3, 20); OW, 90 ± 5, 78–99 (3, 20); OvL, 163 ± 12, 146–186 (1, 8); OvW, 201 ± 9, 190–213 (1, 8); oAL, 50 ± 5, 37–47 (3, 20); oAW, 36 ± 4, 25–31 (3, 20); fAL, 159 ± 17, 135–188 (3, 20); fAW, 69 ± 5, 56–79 (3, 20).
Description: Colony encrusting, laminar to plurilaminar, multiserial. Zooecia hexagonal, separated by shallow grooves. Frontal wall slightly convex, smooth, imperforate except for between three and five small round or slit-like marginal areolar pores; ooecial pore slightly larger, round. Primary orifice slightly longer than wide; anter semicircular, proximal margins very short and sloping, passing into a deep, broad, and widely U-shaped sinus, occupying more than twothirds of the total width, condyles short and blunt; orifice surrounded by a low thickened rim incorporating the oral avicularium.
Ooecium, broader than long, shallowly vaulted, marginal band of ectooecium very narrow, exposed entooecium therefore large, with a smooth surface and 14–16 very low ribs, generally discernible only at the very margin; distinct proximal band of ectooecium paralleling and framing the semicircular aperture.
Small oral avicularia single, lateral to orifice with the crossbar usually located distal to mid-distance, oval, situated at the distal end of a slightly raised cystid, frontal plane at an acute angle to colony surface; rostrum semi-elliptical with a hooked tip, directing laterally or proximolaterally, proximal uncalcified area narrower, semicircular; crossbar complete, without columella. Typical, large, frontal avicularia often present in ontogenetically young zooids, situated lateral to orifice opposite to small avicularium, others may arise from marginal pore anywhere on frontal zooid surface during later ontogeny, located distally on large, slightly swollen, smooth and imperforate cystid; rostrum elongate triangular, narrowing slightly distal to crossbar, downcurved and becoming very slender and parallel-sided distally, oral avicularia point proximally, other frontal avicularia are irregularly orientated; proximal uncalcified area broadly semi-elliptical, distal area relatively small and restricted, and of varying shape; crossbar complete without columella.
Remarks: Although the specimens from Santa Cruz presented here are from Norman’s Collection (NHM), as are the specimens of Buffonellaria jensi sp. nov. from Porto Santo (described below), he did not mention them in his paper on Madeiran bryozoans ( Norman, 1909). Other specimens from Madeira identified as Schizoporella biaperta by Hincks (1880b: 76) are likely to belong to the genus Stephanollona , as Hincks already mentioned, which is evidenced by the presence of oral spines and spatulate avicularia.
Secondary calcification, and therefore variability in colony morphology, is not as prominent in this species compared with most other taxa, e.g. B. jensi sp. nov. Furthermore, because of the characteristic large avicularium situated lateral to the orifice, and because of the large and smooth entooecial area, B. ritae sp. nov. is easily distinguished from all other species.
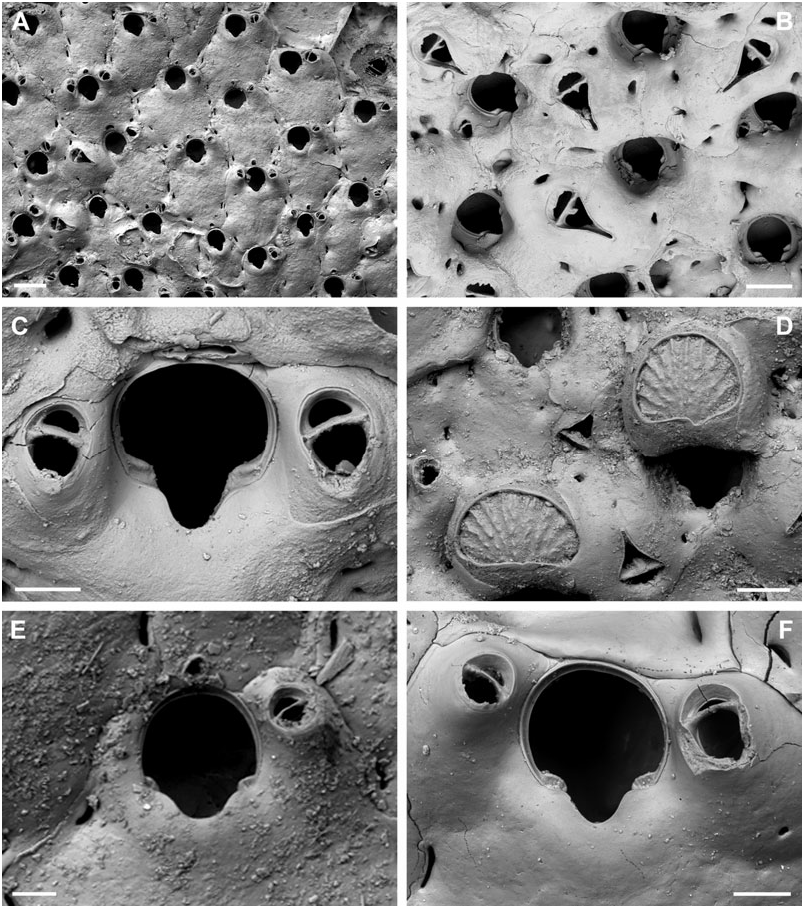
BUFFONELLARIA NEBULOSA ( JULLIEN & CALVET, 1903) View in CoL
( FIG. 5A–E View Figure 5 )
Original description: Hippothoa nebulosa Jullien & Calvet, 1903: 87 , pl. 10, fig. 8.
Material examined: Lectotype: MNHN 3991 About MNHN a, Azores, RV ‘Hirondelle’, Stn 247, 318 m, on octocoral.
Paralectotype: MNHN 3991 About MNHN b, Azores , RV ‘Hirondelle’, Stn 226, 130 m, on a relict celleporid bryozoan .
Measurements: ZL, 629 ± 39, 563–696 (1, 15); ZW, 522 ± 31, 469–583 (1, 15); OL, 145 ± 4, 139–152 (1, 20); OW, 132 ± 7, 121–145 (1, 20); OvL, 295 ± 8, 286– 309 (1, 6); OvW, 333 ± 5, 328–342 (1, 6); oAL, 69 ± 6, 55–76 (1, 12); oAW, 51 ± 5, 43–58 (1, 12); fAL, 185 ± 15, 171–217 (1, 16); fAW, 78 ± 6, 69–88 (1, 16).
Description: Colony encrusting, unilaminar, multiserial. Zooecia quadrate to hexagonal, initially separated by shallow grooves, which are covered by thin layers of secondary calcification during later ontogeny. Frontal wall slightly convex to flat, granular, imperforate except for some three or four small, sickle-shaped, marginal areolar pores; ooecial pore small and round. Primary orifice longer than wide; anter comprising two-thirds of a full circle, proximal margins short and slightly sloping, passing into rounded shoulders of a rounded V-shaped or U-shaped sinus, occupying about two-thirds of the total width, condyles conspicuous, as long as proximal margins and broad, slightly sloping, and with rounded edges.
Ooecia about as broad as long, initially globular, the lateral wall becoming covered by thin layers of secondary calcification during ontogeny, exposed entooecium relatively large, semicircular with a slightly concave proximal border, surface convex with 16–20 distinct, prominent, tubercular ribs meeting and levelling proximomedially, proximal ooecium margin slightly concave.
Small oral avicularia single or paired, distal to mid-distance of orifice, in close proximity of orifice rim, oval, situated at the distal end of a slightly raised and swollen cystid, with a tiny proximal pore, frontal plane at an acute angle to colony surface; rostrum semi-elliptical, directing proximolaterally, proximal uncalcified area semicircular; crossbar complete, possibly with small columella. A single larger avicularium per zooid usually present, developing during early ontogeny, situated proximolateral to oral avicularium, others may arise from marginal pore anywhere on frontal zooid surface during later ontogeny, located distally on a slightly swollen and smooth cystid with a few, small, round pores; rostrum narrowing distal to crossbar, extremely long, thin, and parallel-sided distally, downcurved; frontal avicularia proximal to oral avicularia, point proximally, other frontal avicularia irregularly orientated; proximal uncalcified area semicircular, distal area arrowheadshaped; crossbar complete with a stout bifid columella. Remarks: Both specimens here designated as lectotype and paralectotype were stored in the same small glass vial with a handwritten label identifying them as ‘type’. They also share the same collection number, although they derive from two different sample stations (Stn 247, Stn 226), but it is impossible to identify to which sample each specimen belongs. Therefore, as there are no characteristic elements in the original figure in Jullien & Calvet (1903) that can be related to any of the available type specimens, the larger colony containing ovicells is here designated as lectotype.
Four years after the introduction of this species, Calvet (1907: 423) recorded several specimens of H. nebulosa from the Azores. However, without a description or illustration of the material, it is not possible to judge whether these specimens are synonymous with B. nebulosa because another species ( Buffonellaria acorensis sp. nov., see below) also occurs in this area.
Buffonellaria nebulosa is a deep-water species that has only been reported from the Azores archipelago, in depths of 130– 320 m. In contrast to most other species, B. nebulosa develops distinctly less secondary calcification, and therefore achieves less ontogenetic morphological variability. However, orifice morphology, with the sinus being rather narrow and V-shaped or widely U-shaped, may be subject to considerable intracolonial variability. The formation of a dimorphic avicularium with a determined position on the frontal wall during early ontogeny is shared with B. divergens , as well as with B. ritae sp. nov., B. acorensis sp. nov., and Buffonellaria sp. 2 (see below).
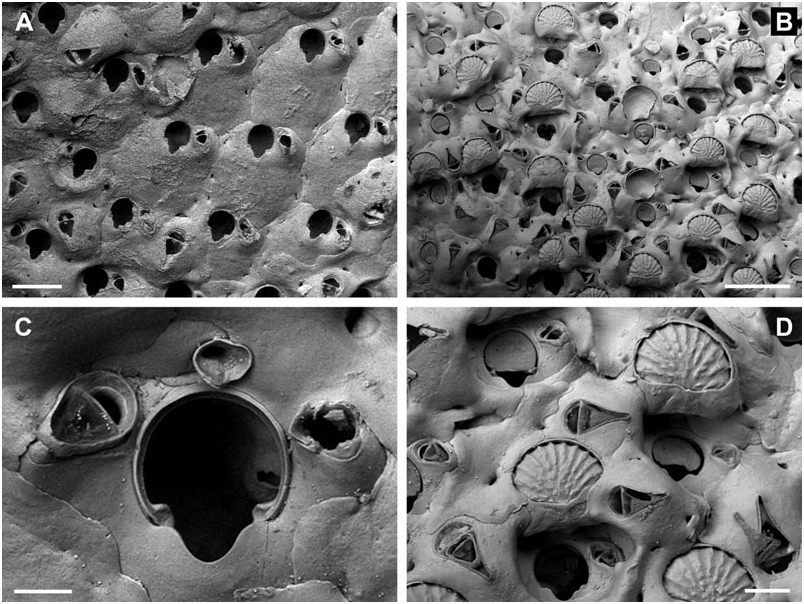
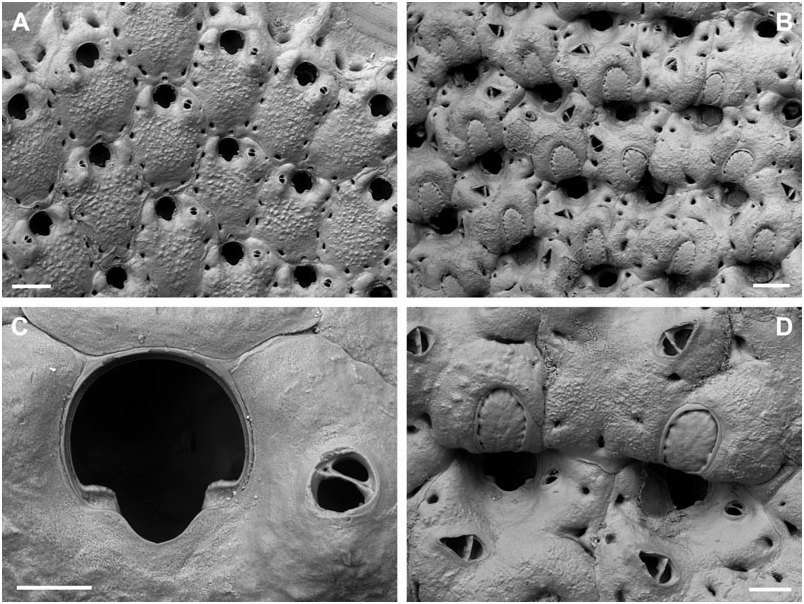
BUFFONELLARIA ACORENSIS SP. NOV.
( FIG. 6A–D View Figure 6 )
Synonyms: Stephanosella biaperta ( Waters, 1879) [sic]: d’Hondt, 1975: 576.
Material examined: Holotype: MNHN BRY-20057 , off São Miguel Island , Azores , RV ‘ Jean Charcot’ , Biaçores Expedition, Stn 145 ( 37°41′N, 25°37.5′W), 148– 135 m, on rock. GoogleMaps
Paratype: MNHN BRY-20058 , off São Miguel Island , Azores , RV ‘ Jean Charcot’ , Biaçores Expedition, Stn 63 ( 38°37.5′N, 28°36.5′W), 220– 165 m, on rock GoogleMaps .
Other material: MNHN 7487 About MNHN , off Flores Island, Azores , RV ‘Jean Charcot’, Biaçores Expedition, Stn 109 ( 39°33′N, 31°17′W), 230– 190 m, on shell GoogleMaps .
Differential diagnosis: B. acorensis sp. nov. has a distinctly greater number of entooecial ribs than any other species, apart from B. nebulosa , from which it differs in having a broader, U-shaped sinus, frontal avicularia with a shorter and broader rostrum, and a more pronounced secondary calcification in ovicellate colony regions. The occasional occurrence of a frontal avicularium with a determined position on the frontal wall further distinguishes this species from B. jensi sp. nov., Buffonellaria muriella sp. nov., Buffonellaria harmelini sp. nov., Buffonellaria antoniettae sp. nov., and Buffonellaria arctica sp. nov (see below). Buffonellaria divergens has distinctly smaller oral and frontal avicularia, with the latter directing laterally.
Etymology: From the type locality, the Azores (Açores in Portuguese).
Measurements: ZL, 533 ± 80, 395–686 (2, 20); ZW, 427 ± 75, 282–529 (2, 20); OL, 131 ± 13, 108–152 (2, 20); OW, 128 ± 14, 104–154 (2, 20); OvL, 239 ± 18, 218–268 (2, 9); OvW, 305 ± 7, 296–320 (2, 9); oAL, 65 ± 8, 48–84 (2, 20); oAW, 47 ± 7, 37–62 (2, 20); fAL, 148 ± 21, 116–200 (2, 20); fAW, 71 ± 9, 56–86 (2, 20).
Description: Colony encrusting, unilaminar, multiserial. Zooecia quadrate to hexagonal, initially separated by shallow grooves. Frontal wall very slightly convex to flat, smooth, imperforate except for some three small, slit-like or sickle-shaped, marginal areolar pores, ooecial pore larger, usually transversely elliptical. Primary orifice slightly longer than wide; anter semicircular, proximal margins short and sloping, passing into rounded shoulders of a widely opening and deep, rounded V-shaped or broadly U-shaped sinus, occupying a little less than twothirds of the total width, condyles conspicuous, short but broad, blunt, very slightly sloping; orifice in ovicellate zooids commonly surrounded by a thickened rim of secondary calcification, with the oral avicularia incorporated into the interior side.
Ooecium broader than long, initially globular, becoming immersed during ontogeny, band of ectooecium relatively narrow, exposed entooecium therefore quite large, semicircular, surface convex with 18–21 distinct, prominent, tubercular ribs meeting proximomedially, proximal ovicell margin occasionally with a prominent rim of secondary calcification.
Small oral avicularia single or paired, lateral to orifice, oval, situated at the distal end of a slightly raised and swollen cystid with a tiny proximal pore, frontal plane at an acute angle to colony surface; rostrum semi-elliptical, directing proximally or proximolaterally, rarely laterally, proximal uncalcified area semicircular; crossbar complete, with small columella. Additional larger avicularia single, sporadic to common, occasionally developing relatively early during ontogeny, usually situated proximolateral to oral avicularium, others may arise from marginal pore anywhere on frontal zooid surface during later ontogeny, located distally on a slightly swollen and smooth cystid, with a small, slit-like, proximal pore; rostrum elongate triangular, variable in length, narrowing distal to crossbar, downcurved, and occasionally becoming very slender and parallel-sided distally, frontal avicularia proximal to oral avicularia point in various proximal directions, other frontal avicularia irregularly orientated; proximal uncalcified area semi-elliptical, distal area arrowhead-shaped; crossbar complete, relatively thick, with stout columella.
Remarks: Although d’Hondt (1975: 576) cites Waters as the authority of Stephanosella biaperta when describing the present specimens, Waters (1879: 37) did not introduce a new species in his paper, but only omitted to state the authority of the species he identified as Lepralia linearis var. biaperta . However, whereas Waters’ specimens closely resemble B. muriella sp. nov. (see below), they differ from B. acorensis sp. nov. in having a deeper sinus, narrower ovicells, and in producing less secondary calcification in colony regions with ovicellate zooids.
Colonies of MNHN 7487 differ from the holotype and paratype in having less marked ovicell ribs and peristomes around orifices of ovicellate zooids. However, as all other characters correspond to those of the holotype, these differences are considered to reflect intraspecific variation. Genetic analyses may falsify this statement. The great variability in zooecium length and width is, to some extent, to the result of growth on an irregular substrate.
It is interesting to note that this is the second species recorded from the fairly isolated Azores archipelago, besides B. nebulosa . Apart from the occurrence of frontal avicularia during early ontogeny in both B. nebulosa and B. acorensis sp. nov., however, they do not seem to be very closely related morphologically. Concerning general zooecium morphology, B. acorensis sp. nov. is rather similar to B. harmelini sp. nov., B. jensi sp. nov., and B. muriella sp. nov. (see below), which makes it likely that B. acorensis sp. nov. and B. nebulosa have independently colonized the Azores from different source regions. Even more intriguing is that there was only a single Buffonellaria species present in each of the continental shelf locations covered in this study, whereas only the Madeiran archipelago also hosts two species ( B. jensi sp. nov. and B. ritae sp. nov.).
BUFFONELLARIA JENSI SP. NOV.
( FIG. 7A–D View Figure 7 )
Synonyms: Schizoporella biaperta ( Michelin, 1848) var. divergens ( Smitt, 1873) : Norman, 1909: 303, pl. 40, figs 3, 4.
Material examined: Holotype: NHM 2007.5.18.8, off Porto Santo, Madeira, on the benthic foraminifer M. miniacea .
Paratype: NHM 2007.5.18.9, off Porto Santo, Madeira, colony occurring free of a substrate.
Other material: NHM 1911.10.1.1080, off Porto Santo, Madeira, on M. miniacea and organic substrate.
Differential diagnosis: B. jensi sp. nov. is distinguished from B. divergens , B. nebulosa , B. ritae sp. nov., and B. acorensis sp. nov., in the absence of an early ontogenetic frontal avicularium, with a determined position on the frontal wall, as well as by a much more pronounced secondary calcification of colony regions with ovicellate zooecia. It differs from B. muriella sp. nov. in having a greater number of entooecial ribs, a relatively shorter rostrum in frontal avicularia, and a generally shallower and broader sinus (although sinus shape is subject to considerable variation in B. muriella sp. nov., see below). Differences between B. jensi sp. nov. and B. harmelini sp. nov. are found in the presence of more conspicuous marginal pores, a broader sinus, elliptical oral avicularia, a greater number of entooecial ribs, and in thicker secondary calcification. Buffonellaria antoniettae sp. nov. has distinctly larger and elongate triangular oral avicularia, whereas B. arctica sp. nov. has a granular frontal wall, smaller oral and frontal avicularia, as well as larger ovicells, with an elliptical entooecial area. Buffonellaria jensi sp. nov. also differs from B. porcellanum in having a smooth frontal wall, larger zooecia oral avicularia, and ovicells with a greater number of entooecial ribs, as well as in developing frontal avicularia during later ontogeny.
Etymology: Named in honour of the senior author’s father, Jens Berning.
Measurements: ZL, 549 ± 43, 492–645 (1, 20); ZW, 387 ± 65, 278–537 (1, 20); OL, 127 ± 5, 116–135 (1, 20); OW, 129 ± 8, 117–141 (1, 20); OvL, 208 ± 20, 176–229 (1, 9); OvW, 232 ± 22, 191–264 (1, 9); oAL, 84 ± 8, 70–97 (1, 14); oAW, 61 ± 5, 53–71 (1, 14); fAL, 150 ± 10, 131–167 (1, 7); fAW, 92 ± 8, 77–107 (1, 7).
Description: Colony encrusting, unilaminar, multiserial. Zooecia quadrangular, initially separated by distinct sutures, soon covered by secondary calcification. Frontal wall very slightly convex, smooth, imperforate except for between four and seven small, oval to slit-like, marginal areolar pores, and a few tiny central pseudopores; ooecial pore slightly larger, transversely elliptical. Primary orifice on average about as long as wide, but length/width relationship variable; anter almost two-thirds of full circle, proximal margins short and slightly sloping, passing into rounded shoulders of a deep, broad, and widely U-shaped sinus, occupying a little less than twothirds of the total width, condyles conspicuous, short but broad, blunt; orifice encircled by a thickened rim of secondary calcification during later ontogeny, incorporating the oral avicularium.
Ooecium broader than long, initially globular, becoming immersed during ontogeny, band of ectooecium relatively narrow, exposed entooecium therefore quite large, semicircular, surface slightly convex with 13–17 prominent tubercular ribs meeting proximomedially.
Small oral avicularia single, occasionally paired, lateral to orifice, oval, situated at the distal end of a slightly swollen and raised cystid with a small proximal areolar pore, frontal plane at an acute angle to colony surface; rostrum semi-elliptical, with a few low teeth distally, directing proximolaterally, proximal uncalcified area semicircular; crossbar complete, without columella. Additional larger avicularia frequently present in older parts of the colony, arising from a marginal pore anywhere on frontal zooid surface, located distally on large, slightly swollen, smooth cystid with few marginal areolar pores; rostrum elongate triangular, narrowing distal to crossbar, slightly downcurved, occasionally becoming very slender and parallel-sided distally, pointing in various directions; proximal uncalcified area semicircular, distal area arrowhead-shaped; crossbar complete with a median columella.
Remarks: The original illustration of these specimens published by Norman (1909) differs somewhat from the SEM photos presented here. Although he showed three autozooids with a pair of oral avicularia, only a single one is generally present. Another zooid has a long, distally directed, curved, and pointed oral avicularium, which was not observed to occur in these or other specimens. It may therefore be a broken cystid of an oral avicularium. Moreover, the sinus is somewhat deeper but narrower in Norman’s drawings. However, the drawings are relatively simple, and other material from Madeira (see B. ritae sp. nov.), with which these specimens may have been mistaken, does not match this illustrated specimen either. As the sample location, as well as the note on the original label, which states that Senhor De Noronha donated the specimens, matches with Norman’s (1909: 303) remarks, these specimens are chosen as types, in spite of these inconsistencies.
The co-occurrence of B. ritae sp. nov. and B. jensi sp. nov. around the Madeiran archipelago is, as the co-occurrence of B. nebulosa and B. acorensis sp. nov. around the Azores, worth mentioning because from all other locations studied, only a single species was recorded. In addition, morphological differences are even more pronounced in B. ritae sp. nov. and B. jensi sp. nov. than in the Azores species, thus indicating independent colonization of the islands from different source regions, rather than in situ speciation after a single invasion event.
Whereas hypercalcification is pronounced in ovicellate colony regions in B. jensi sp. nov., profoundly changing the surface morphology, orifice variability is comparatively low. Unfortunately, the depth of occurrence around Madeira is not known. It was found growing on the encrusting foraminifer M. miniacea and on soft organic tissue.
BUFFONELLARIA MURIELLA SP. NOV.
( FIGS 1A View Figure 1 , 2A View Figure 2 , 8A–F View Figure 8 )
Synonyms: Schizoporella biaperta ( Michelin, 1848) : Hincks, 1880: 255, pl. 40, figs 7–9.
Buffonellaria divergens ( Smitt, 1873) View in CoL : Hayward & Ryland, 1979: 204, fig. 86; Hayward & Ryland, 1999: 356, fig. 167; fig. 183C, D (as Stephanollona armata View in CoL ).
Material examined: Holotype: NHM 2007.5.18.1, Guernsey, on bivalve, illustrated by Hayward & Ryland (1979: fig. 86).
Paratypes: NHM 2000.12.5.10, Greenwich Light , Western Channel , Stn 26A, on small pebble; NHM 1897.5.1.726, Hastings, UK, on pectinid, illustrated by Ryland (1969: fig. 2C) .
Other material: NHM 1911.10.1.1082–1083, Guernsey, on bivalves; NHM 2007.5.18.2, Guernsey, on bivalves; NHM 2007.5.18.3, Adriatic Sea, off Puglia, Italy, occurring free of a substrate; CNHM Inv.br.27, off Lastovo , Adriatic Sea, Croatia, 10–15 m, on Halimeda tuna ( Ellis & Solander, 1786) ; CNHM Inv.br.28, off Lastovo , Adriatic Sea, Croatia, 30–40 m, on noncalcifying algae and occurring free of a substrate ; CNHM Inv.br.29, on Jabuka Shoal , Adriatic Sea, Croatia, 40–50 m, on Pentapora fascialis ( Pallas, 1766) , as well as on coralline and other noncalcifying algae .
Differential diagnosis: B. muriella sp. nov. lacks early ontogenetic frontal avicularia with a determined position on the frontal wall, and can therefore be distinguished from B. divergens , B. ritae sp. nov., B. nebulosa , and B. acorensis sp. nov., which also produce distinctly less secondary calcification in colony regions with ovicellate zooecia. Buffonellaria muriella sp. nov. differs from the morphologically closely related B. jensi sp. nov. in having a generally narrower and deeper sinus, with longer condyles (but see below), fewer entooecial ribs, and a relatively longer rostrum in frontal avicularia. Differences between B. muriella sp. nov. and B. harmelini sp. nov. are found in more conspicuous marginal pores, the smaller and elliptical oral avicularium, and in a slightly more extensive calcification of colony regions with ovicellate zooecia. In contrast to B. antoniettae sp. nov., B. muriella sp. nov. has smaller zooecia, fewer entooecial ribs, and distinctly smaller elliptical avicularia. Buffonellaria muriella sp. nov. differs from B. arctica sp. nov. in having a smooth frontal wall, a semicircular entooecial area, and a larger frontal avicularia. It is also distinguished from B. porcellanum by the smooth frontal surface, as well as by the presence of frontal avicularia, and larger zooecia, orifices, ooecia (with a greater number of entooecial ribs), and oral avicularia, which are situated lateral to the orifice.
Etymology: Named after a good friend of the senior author, Muriel Kock.
Measurements: ZL, 526 ± 51, 458–626 (2, 20); ZW, 366 ± 36, 299–431 (2, 20); OL, 140 ± 8, 118–153 (2, 20); OW, 124 ± 7, 105–135 (2, 20); OvL, 217 ± 36, 174–261 (2, 8); OvW, 248 ± 34, 190–284 (2, 8); oAL, 67 ± 11, 53–96 (2, 20); oAW, 54 ± 6, 44–65 (2, 20); fAL, 154 ± 13, 130–180 (2, 20); fAW, 86 ± 8, 67–101 (2, 20).
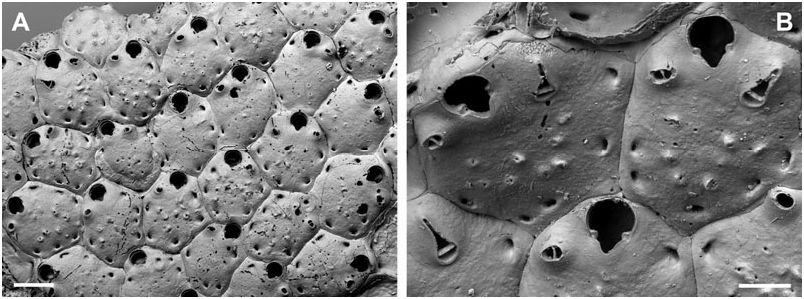
Description: Colony encrusting, unilaminar to plurilaminar, multiserial, occasionally erect bilaminar (observed in material from the Adriatic Sea only). Zooecia hexagonal to elongate hexagonal, quadrangular, or oval, separated by shallow grooves that become obscured by secondary calcification during later ontogeny; frontal wall slightly convex, smooth, initially pseudoporous, becoming imperforate or with only tiny pseudopores remaining during later ontogeny; between four and seven slit-like, marginal, areolar pores; ooecial pore rimmed, round or oval. Primary orifice variable in shape, and therefore in relation of length and width, usually longer than wide; anter more or less semicircular, proximal margins short, straight, and slightly sloping, passing into rounded shoulders of poster, varying in shape from generally deep and rounded V-shaped to relatively shallow and broadly U-shaped, occupying more than half of the total width; condyles variable in shape and size, usually conspicuous, relatively broad, blunt, more or less paralleling the proximal margin, occasionally smaller and shorter than the proximal margins.
Ooecium initially globular, prominent, slightly broader than long, frontal semicircular in outline during later ontogeny when it becomes almost completely immersed by secondary calcification; ectooecium a broad smooth band, exposed entooecium semicircular, relatively flat, marked with 11–13 radiating tubercular ribs slightly thickening towards margin.
Small oral avicularia single or paired, usually proximolateral or lateral to orifice, occasionally distal to mid-distance of orifice, oval, situated on a slightly raised and swollen cystid, with a tiny, proximal, areolar pore, frontal plane at an acute angle to colony surface; rostrum semi-elliptical, directing proximally or proximolaterally (rarely laterally), distally toothed and slightly raised; proximal uncalcified area semicircular; crossbar complete, occasionally with a small columella. Additional larger avicularia present in older areas of the colony, arising from marginal pores anywhere on frontal zooid surface, situated on large swollen cystid with marginal pores and a smooth surface; rostrum elongate triangular, narrowing distal to crossbar, becoming very slender and parallelsided distally, pointing in various directions; proximal uncalcified area semicircular; crossbar complete with a median columella.
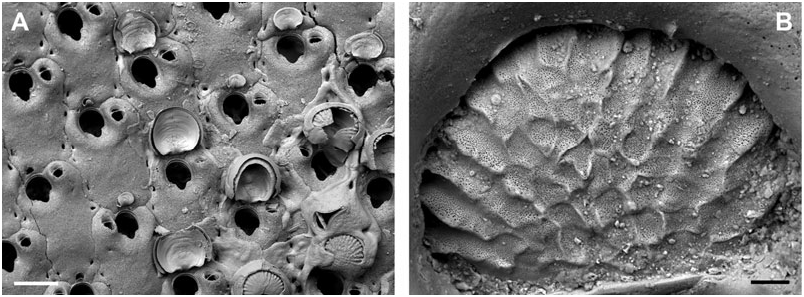
Remarks: Apart from colony growth type, the species description of B. muriella sp. nov. is based upon specimens from off south-east England only. Although there are no differences in discrete features between specimens from the north-east Atlantic and the Adriatic Sea, the latter are characterized by generally having somewhat smaller condyles ( Fig. 8F View Figure 8 ), and slightly larger oral [oAL, 85 ± 10, 68–103 (3, 17); oAW, 66 ± 8, 55–85 (3, 17)] and frontal avicularia [fAL, 176 ± 16, 150–197 (2, 15); fAW, 90 ± 6, 82–102 (2, 15)], whereas zooecium, orifice, and ooecium sizes are all identical. Furthermore, Adriatic specimens have a greater number of ovicell ribs (15–17, see Fig. 2A View Figure 2 ), a less conspicuous columella in frontal avicularia, and, as mentioned above, occasionally exhibit erect bilaminar growth, which was not observed in north-east Atlantic specimens. However, we do not consider these differences a strong enough argument to separate these morphotypes into two species, because we cannot rule out local environmental conditions or intraspecific genetic differences between populations as causal mechanisms, especially considering the presence of strong intracolonial variability (see below). Genetic analyses are certainly needed to clarify the relationship between these populations.
Although encrusting and erect parts of the colony in Adriatic specimens did not show any differences in zooecium morphology, this species stands out by its extreme variability in surface topography, which is, to a great extent, produced by secondary calcification during ontogeny. More importantly, the orifice shape is also prone to considerable variation, and, especially in the British specimens, may change from deep and rather V-shaped to shallow and broadly U-shaped within a single colony, approaching orifice shapes displayed in the closely related B. jensi sp. nov. and B. harmelini sp. nov. In addition, zooecium shape may vary between broadly hexagonal or quadrangular and relatively elongated, with a narrow proximal region cramped by the proximal zooids. Under these conditions, defining species boundaries in this genus may occasionally prove to be a contentious issue.
During early ontogeny the frontal wall is, as Hincks (1880a: 255) already noted, centrally perforated by relatively large pseudopores ( Fig. 1A View Figure 1 ). These are either rapidly closed by secondary calcification or remain present as tiny pseudopores, even during later ontogeny (see also B. porcellanum , as well as Buffonellaria sp. 1 and Buffonellaria sp. 2 below). Another feature observed in specimens from the Adriatic was that in the zooid distal to a maternal zooid, secondary calcification of the proximal frontal wall around the ooecial pore was suppressed, forming a shallow bowl in which the ooecium fits into at a later stage.
As it is unclear how many different Atlantic and Mediterranean species were lumped together under the former name B. divergens , very little certain information exists about the ecology of B. muriella sp. nov. Most of the specimens from the north-east Atlantic encrust pectinids and other bivalves. Colonies from the Adriatic Sea were observed to grow on H. tuna , corallinaceans, and fleshy algae, and the erect foliaceous bryozoan P. fascialis in depths of 10–50 m (M. Novosel, pers. comm. 2006). Erect growth was observed to occur in specimens of only one sample ( CNHM Inv.br. 28, 30–40 m), whereas the substrate was not preserved and therefore was presumably of ephemeral nature.
The presently affirmed range of geographical distribution of the north-east Atlantic populations of B. muriella sp. nov. extends from the Irish Sea (H. de Blauwe, pers. comm. 2006) via Guernsey to the eastern English Channel. In the Mediterranean Sea, B. muriella sp. nov. was recorded in southern Croatia and south-east Italy, and possibly also occurs along the south-west Italian coast as far as Naples, as Waters’s (1879: 37, pl. 11, figs 1, 2) specimens closely resemble this species. Interestingly, in the Adriatic Sea its occurrence is restricted to the southern part, as it was not recorded by Hayward & McKinney (2002), who have thoroughly sampled the north-east Adriatic region off Croatia. However, minimum temperatures and mean annual temperatures in the northern part of the Adriatic Sea are comparable with those off south-west England, whereas temperatures in the southern Adriatic are distinctly higher (see Novosel, Pozar-Domac & Pasaric, 2004; Lombardi et al., 2006). Thus, if the distribution of the Adriatic population is controlled by temperature, this may indicate that populations from southern England and the Adriatic Sea have different environmental preferences, and may, indeed, be different taxa.
BUFFONELLARIA HARMELINI SP. NOV.
( FIG. 9A–D View Figure 9 )
Material examined: Holotype: NHM 2007.5.18.10, Gabinière islet, Port-Cros Island, Mediterranean Sea ( 42°53.16′N, 6°23.5′E), 58– 50 m, on Myriapora truncata ( Pallas, 1766) . GoogleMaps
Paratype: NHM 2007.5.18.11, Gabinière islet, Port-Cros Island, Mediterranean Sea ( 42°53.16′N, 6°23.5′E), 58– 50 m, occurring free of a substrate GoogleMaps .
Differential diagnosis: B. harmelini sp. nov. differs from all but one of the other species in having a larger oral avicularium with a triangular rostrum. The only exception is B. antoniettae sp. nov., which has triangular avicularia, but ones that are more elongated and therefore even larger than those in B. harmelini sp. nov., and which forms erect bilaminar colonies.
Etymology: Named after our appreciated colleague and collector of the specimens, Jean-Georges Harmelin.
Measurements: ZL, 470 ± 39, 396–552 (1, 18); ZW, 350 ± 29, 305–410 (1, 18); OL, 142 ± 10, 122–153 (1, 19); OW, 122 ± 7, 109–132 (1, 19); OvL, 213 ± 12, 187–239 (1, 13); OvW, 254 ± 18, 219–291 (1, 13); oAL, 88 ± 9, 72–104 (1, 14); oAW, 57 ± 6, 44–67 (1, 14); fAL, 172 ± 25, 140–219 (1, 20); fAW, 94 ± 12, 76–119 (1, 20).
Description: Colony encrusting, unilaminar, multiserial. Zooecia rectangular in early ontogeny, separated by shallow grooves, soon altered by secondary calcification during ontogeny. Frontal wall slightly convex, initially even and smooth, imperforate except for between three and six small slit-like or sickle-shaped marginal areolar pores during early ontogeny; ooecial pore conspicuous, generally round. Primary orifice slightly longer than wide; anter two-thirds of a full circle, proximal margins short, straight or slightly sloping, passing into rounded shoulders of a variably deep, U-shaped to rounded V-shaped sinus, occupying about two-thirds of the total width, condyles short but distinct, blunt.
Ooecium initially globular, frontal rather hemispherical during later ontogeny when marginally covered by secondary calcification, wider than long; ectooecium a broad smooth band encircling a slightly convex, semicircular, frontal area of uncovered entooecium, marked by between nine and 13 distinct, tubercular, radiating ribs meeting proximomedially.
Oral avicularia single, rarely paired, lateral to orifice, with the crossbar located at mid-distance or slightly distal to it, oval, situated on a slightly raised cystid, frontal plane at an acute angle to colony surface; rostrum rounded triangular with a hooked tip, directing proximolaterally, proximal uncalcified area semicircular; crossbar complete, without columella. Additional larger avicularia present in older areas of the colony, arising from marginal pore anywhere on frontal zooid surface, situated on large swollen cystid with marginal pores and a smooth surface; rostrum elongate triangular, narrowing distal to crossbar, becoming very slender and parallelsided distally, pointing in various directions, occasionally downcurved distally; proximal uncalcified area semicircular, triangular distally; crossbar complete with a median columella.
Remarks: As in most other species, the orifice shape is quite variable in B. harmelini sp. nov., but secondary calcification in ovicellate colony regions does occur, but is not as extreme as in, e.g. B. antoniettae sp. nov. (see below). Apart from the rounded triangular oral avicularium, B. harmelini sp. nov. is morphologically closely related to B. acorensis sp. nov. (this species differs in having a greater number of entooecial ribs and in producing frontal avicularia during early ontogeny), as well as to B. jensi sp. nov. and to B. muriella sp. nov. (both of which have more conspicuous marginal pores and produce more extensive frontal calcification during later ontogeny). Another species with a triangular oral avicularium of intermediate size between B. harmelini sp. nov. and B. antoniettae sp. nov. is Buffonellaria sp. 1 , which also occurs in the north-west Mediterranean Sea, and is discussed below.
The colonies often show repaired avicularia ( Fig. 10D View Figure 10 ). Commonly, the large frontal avicularia are then replaced by smaller oral-type ones. The geographical range of B. harmelini sp. nov. within the Mediterranean Sea is not known, as descriptions and illustrations of other records of Buffonellaria are insufficient for a precise determination (e.g. Gautier, 1962: 155; Zabala & Maluquer, 1988: 127, fig. 286).
( FIGS 1B View Figure 1 , 10A, B View Figure 10 )
Material examined: NHM 2007.5.18.12, off Toulon, Grande Rade, Mediterranean Sea, 53 m, occurring free of a substrate.
Measurements: ZL, 655 ± 80, 533–738 (1, 20); ZW, 350 ± 34, 262–404 (1, 20); OL, 156 ± 8, 143–167 (1, 16); OW, 123 ± 4, 118–135 (1, 16); oAL, 120 ± 11, 104–138 (1, 15); oAW, 64 ± 5, 54–71 (1, 15).
Description: Colony erect, bilaminar, foliaceous. Zooecia elongate rectangular, usually narrower proximally, initially separated by sutures on thin ridges, zooecial boundaries become wavy later, and are obscured by secondary calcification. Frontal wall with up to eight marginal areolar pores, and several pseudopores during early ontogeny, most of which soon become closed by secondary calcification, some tiny pores occasionally persist during later ontogeny, surface slightly convex, smooth. Primary orifice slightly longer than wide; anter about two-thirds of a full ellipse, proximal margins short and sloping, passing into a broad, deep, U-shaped sinus, occupying about two-thirds of the total width, condyles broad, blunt, more or less paralleling the proximal margins.
Ovicells were not observed.
Oral avicularia single, situated lateral to orifice, slightly distal to mid-distance of orifice, on a proximally raised cystid with a small proximal pore; rostrum triangular, directing proximolaterally, acute to frontal plane; crossbar complete, without columella, proximal uncalcified area more or less semicircular. Additional frontal avicularia were not observed.
Remarks: As no ovicells and larger frontal avicularia are present in the only colony fragment available for study, we refrain from introducing a new species. Concerning general zooecial morphology and size, Buffonellaria sp. 1 is very closely related to B. antoniettae sp. nov. (see below), and is, besides B. antoniettae sp. nov. and Adriatic specimens of B. muriella sp. nov., the only other species with an erect bilaminar branching or foliaceous growth form encountered in this study. The erect growth type is therefore only observed in taxa from the Mediterranean Sea. Without being able to compare ooecium morphology, B. antoniettae sp. nov. differs from Buffonellaria sp. 1 in a slightly more proximally positioned oral avicularium, with a significantly longer and distally thinner rostrum, as well as a slightly thicker crossbar. In addition, the sinus is generally deeper and more V-shaped in B. antoniettae sp. nov. Furthermore, orifice and oral avicularium morphology are also almost identical to those of B. harmelini sp. nov. However, the latter was only observed to form unilaminar encrusting colonies, has significantly smaller zooecia and oral avicularia, and has less conspicuous marginal pores. Although we cannot rule out the possibility that erect growth also occurs in B. harmelini sp. nov., the differences in several characters make it unlikely that Buffonellaria sp. 1 belongs to either B. antoniettae sp. nov. or B. harmelini sp. nov.
BUFFONELLARIA ANTONIETTAE SP. NOV.
( FIGS 2B View Figure 2 , 11A–F View Figure 11 )
Material examined: Holotype: PMC B16.30.6.2006a, off south-east Sicily, ‘ Vega’ 9, 118 m, colony fragment occurring free of a substrate.
Paratypes: PMC B16.30.6.2006b, off south-east Sicily, ‘ Vega’ 9, 118 m, three colony fragments occurring free of a substrate .
Differential diagnosis: B. antoniettae sp. nov. differs from all other known and newly introduced species in its erect bilaminar growth form, and its large, elongate triangular, oral avicularium.
Etymology: Named after our appreciated colleague and collector of the specimens, Antonietta Rosso.
Measurements: ZL, 627 ± 66, 504–731 (2, 20); ZW, 411 ± 33, 316–450 (2, 20); OL, 151 ± 6, 139–160 (2, 20); OW, 127 ± 6, 116–141 (2, 20); OvL, 209 ± 9, 198–223 (1, 15); OvW, 263 ± 11, 247–286 (1, 15); oAL, 178 ± 10, 161–198 (3, 15); oAW, 81 ± 6, 72–96 (3, 15); fAL, 194 ± 17, 149–219 (2, 12); fAW, 111 ± 9, 98–126 (2, 12).
Description: Colony erect, bilaminar branching, branches some 3-mm wide. Zooecia elongate hexagonal, quadrangular, or oval, separated by indistinct meandering sutures; frontal wall slightly convex, imperforate and smooth, but initially with few pits and tiny pseudopores that soon become obscured by secondary calcification; initially between five and ten round or elongate, relatively small, marginal, areolar pores, and in some zooecia a round rimmed ooecial pore of approximately the same size. Primary orifice variable in shape; anter about two-thirds of a full circle, proximal margins short and sloping, condyles short, but broad and slightly sloping, blunt, median margin straight, poster generally deep, broad, and a rounded V-shape, occasionally shallower and rather U-shaped.
Ooecium initially globular, slightly flattened frontally, central exposed entooecium large, semicircular, first with a relatively narrow proximal band of ectooecium, later slightly immersed by a thick rim of secondary calcification; surface of entooecium with 15–17 distinct, thin, radiating ribs, occasionally fusing towards the centre forming a meshwork, proximal margin straight in frontal view.
Oral avicularia large, single, occasionally absent (especially in zooids at branch margins), situated directly lateral to orifice on a broad and very slightly raised cystid that has a tiny proximal pore, frontal plane at an acute angle to colony surface; rostrum elongate triangular, directing proximolaterally, distal tip slightly downcurved; uncalcified area semicircular proximally, elongate triangular distally; crossbar complete, without columella; oral avicularia are completely covered by secondary calcification in ovicellate colony regions. Additional larger avicularia present in older areas of the colony, arising from marginal pore anywhere on frontal zooid surface, situated on large swollen cystid, with marginal pores and a smooth surface; rostrum elongate triangular, narrowing distal to crossbar, becoming very slender and parallelsided distally, pointing in various directions, occasionally slightly downcurved distally; uncalcified area semicircular proximally, rounded triangular distally; crossbar complete with a median columella.
In older ovicellate parts of the colony, hypercalcification completely covers the oral avicularia and deeply immerses the orifice; the frontal is then composed of larger areolar pores, the slightly immersed entooecial area, frontally budded avicularia, and semicircular, round or irregular apertures.
Remarks: B. antoniettae sp. nov. is easily distinguished from the other European Buffonellaria species by its large oral avicularia. Furthermore, it has well-developed and bifurcating branches, whereas most other species are mainly or solely encrusters, and seldom form organized bilaminar branches, such as the Adriatic specimens of B. muriella sp. nov. The fragments of dead colonies, here chosen as types, are from a suite of specimens that were recovered from off of south-east Sicily, and the geographical distribution is therefore restricted to this region at present. Although always in subordinate numbers, B. antoniettae sp. nov. was present in all 18 samples taken from muddy to silty sediments, ranging in depth between 115 and 130 m, and co-occurring in this thanatocoenosis with some 20 other (mostly erect) bryozoan species ( Rosso, 1989). However, information on early astogeny, the initial (presumably) encrusting stage, as well as regarding the overall colony shape, is unfortunately not available, although we believe that change of growth type is not likely to have a great effect on zooecium morphology (see Remarks in B. muriella sp. nov.). Hypercalcification in ovicellate colony regions during later ontogeny is conspicuous in this species, completely covering the oral avicularia, and deeply immersing the orifices.
BUFFONELLARIA ARCTICA SP. NOV.
( FIG. 12A–D View Figure 12 )
Synonyms: Schizoporella biaperta ( Michelin, 1848) : Nordgaard, 1906: 15, pl. 1, figs 12–14; Kluge, 1975: 579, fig. 320; Gostilovskaya, 1978: 188, fig. 112.
Material examined: Holotype: NHM 2007.5.18.4, Spitsbergen, no locality details, on balanid plate.
Paratype: NHM 2007.5.18.5, Grey Hook, Spitsbergen, 165 m, on rock.
Other material: NHM 1911.10.1.1073, Greenland, no locality details, 110 m; NHM 1911.10.1.1075, Grey Hook, Spitsbergen, 165 m, two colonies on rocks; NHM 1911.10.1.1079, Greenland, no locality details, on bivalve shells; NHM 1911.10.1.1081, Spitsbergen , no locality details, four colonies on rocks and balanid plates; NHM 2007.02.02.1, Kongsfjorden , Spitsbergen , on rock; CMN (2006)-0004, Nunavut, Forbes Sound , Canada, Calanus Expedition , 25 July 1962 ( 60°22′N, 69°26′W), Powell Collection ; SMNH 1742 View Materials , Edge Island (Storfjord, Whalers Point), Svalbard, Swedish Arctic Expedition 1864, Stn 6 ( 77°20′N, 20°30′E), 55–73 m; illustrated by Smitt (1868: pl. 24, figs 71–73) GoogleMaps .
Differential diagnosis: B. arctica sp. nov. differs from all other species in having a distinctly elliptical area of exposed entooecium. Granulation of the frontal wall is also much more distinct in this species, and the marginal pores are larger and/or more numerous than in the previously described species ( B. porcellanum has larger but fewer pores).
Etymology: For its wide geographical distribution in Arctic waters.
Measurements: ZL, 583 ± 40, 503–656 (2, 20); ZW, 441 ± 43, 366–534 (2, 20); OL, 129 ± 6, 122–141 (2 20); OW, 127 ± 6, 114–137 (2, 20); OvL, 297 ± 23, 236–324 (2, 16); OvW, 300 ± 39, 206–344 (2, 16); oAL, 57 ± 4, 51–69 (2 20); oAW, 46 ± 5, 38–59 (2, 20); fAL, 111 ± 11, 96–134 (2, 20); fAW, 70 ± 6, 60–86 (2, 20).
Description: Colony encrusting, unilaminar, multiserial. Zooecia oval to quadrate or hexagonal, initially separated by shallow grooves, disguised by secondary calcification during ontogeny, as are most other frontal characteristics; distolateral vertical walls with between five and eight relatively large, widely spaced, distolateral communication pores, separated by broad and thick calcification. Frontal wall slightly convex, coarsely granular, imperforate, except for beween five and eight round or oval marginal areolar pores, ooecial pore of about same size, round or oval, rimmed. Primary orifice about as long as wide; anter two-thirds of a full circle, proximal margins short and slightly sloping, passing into a variably broad, deep and widely U-shaped sinus, occupying more than half of the total width, condyles conspicuous, variable, generally short but broad, square, blunt; orifice flanked laterally by a slightly swollen, rather smooth and elongated hump, one or each carrying an oral avicularium.
Ooecium variable in outline, on average as long as wide, initially globular, later immersed by thick, broad, and bulging secondary calcification around the margin; ectooecium extensive, exposed entooecium usually (semi) elliptical, surface relatively smooth with 12–16 very low ribs, usually confined to the very margin.
Oral avicularia single or paired, oval, lateral or proximolateral to orifice at midway to zooid margin, and on a slightly raised cystid with one or two small areolar pores, frontal plane at an acute angle to colony surface; rostrum semi-elliptical, directing proximally or proximomedially, proximal uncalcified area narrower and more or less semicircular; crossbar complete, without columella. Additional avicularia frequent, budding from marginal pore anywhere on frontal during later ontogeny, situated on a swollen cystid, surface smooth and imperforate, except for two areolar pores; rostrum triangular, relatively short, with a blunt tip, pointing in various directions; proximal uncalcified area more or less semicircular, distal area rounded triangular; crossbar complete without columella, but occasionally slightly thickened centrally.
Remarks: The confusion associated with the use of Michelin’s name biaperta for modern Arctic Buffonellaria dates back to 1868 when Smitt, assuming his specimens were synonymous with the Pliocene species that Busk (1859: 47, pl. 7, fig. 5) identified as (?) Lepralia biaperta , applied this name to a recent species for the first time ( Smitt, 1868: 14, pl. 24, figs 70–73). However, although Ryland (1969: 220, fig. 2A) showed that Michelin’s fossil taxon is not only a distinct species, but also belongs to a different genus, authors have continued to name Arctic specimens biaperta to date.
Smitt (1868) originally illustrated two specimens as Escharella linearis ( Hassall, 1841) forma biaperta ( Michelin, 1848) , one with autozooids covered by extensive secondary calcification ( Smitt, 1868: figs 71–73; SMNH 1742), which we examined using SEM ( Fig. 13A, B View Figure 13 ), and one with autozooids lacking frontal calcification ( Smitt, 1868: fig. 70). Neither specimen contains ovicells; however, we decided not to choose these specimens as types for B. arctica sp. nov. for two reasons. First, specimen SMNH 1742 differs from all other studied material in having distinctly larger marginal pores, as well as non-ovicellate zooids that are covered by thick and bulgy secondary calcification. Although the size of the marginal pores was to some extent also variable in the types chosen here, and in other specimens, these marginal pores were, generally, distinctly smaller, and secondary calcification was only observed in ovicellate zooids. Second, we were not allowed to clean specimen SMNH 1742. Comparison of important subcuticular characters (such as condyles) was therefore impossible. Thus, we cannot rule out the possibility that two species exist in the Arctic, and we cautiously decided not to synonymize Smitt’s material with B. arctica sp. nov.
Nevertheless, all studied specimens of B. arctica sp. nov. are morphologically consistent throughout, despite displaying general variability in orifice morphology, and intracolonial differences in sinus and condyle shape, which seems to be typical in Buffonellaria . Owing to thick calcification around ovicells, colony regions with ovicellate zooids have a conspicuously bulgy topography during later ontogeny, with interspersed, typically elliptical areas of exposed entooecia.
Material examined with SEM comprises specimens from Spitsbergen and Greenland. Buffonellaria arctica sp. nov. thus has, compared with most other species presented here, a fairly wide geographical range of distribution. Although not observed with SEM, specimens from north-east Canada ( CMN 2006- 0004; see Nordgaard, 1906; Powell, 1968) and the figures of specimens from north-west Russia ( Kluge, 1975; Gostilovskaya, 1978) do not show any significant differences, therefore supporting this scenario. Whether further records from the Laptev Sea, the Sea of Okhotsk ( Kluge, 1975; Dahle et al., 1998), the Sea of Japan ( Androsova, 1958: 132, fig. 52), and the Pacific coasts of North America (e.g. Osburn, 1952: 368, pl. 42, figs 1, 2) indeed belong to this taxon is yet to be proven.
In its type area, the archipelago of Svalbard, B. arctica sp. nov. is a fairly rare species, as it occurs in only 2% of the samples collected by Kuklinski (2002). It encrusts shells and rocks in depths between 40 and 200 m ( Kuklinski, 2002; Kuklinski et al., 2005), which is in agreement with records from other Arctic regions, where it was also observed to grow on hydroids and calcareous algae (e.g. Kluge, 1975). Kluge furthermore reported this species to grow in water temperatures of down to -1.3 °C.
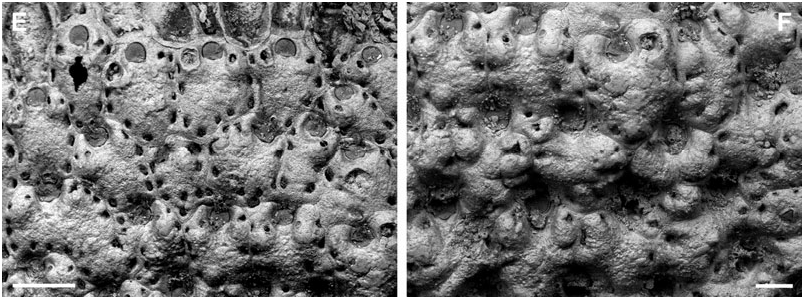
BUFFONELLARIA PORCELLANUM ARÍSTEGUI RUIZ, 1987
( FIG. 14A–D View Figure 14 )
Original description: Buffonellaria porcellanum Arístegui Ruiz, 1987: 530 , figs 4, 10–14.
Material examined: Holotype: NHM 1987.1.3.2, Playa los Cancajos, La Palma, Canary Islands.
Measurements: ZL, 427 ± 65, 345–590 (1, 20); ZW, 318 ± 40, 263–396 (1, 20); OL, 92 ± 4, 86–97 (1, 15); OW, 92 ± 5, 84–104 (1, 15); OvL, 147 ± 14, 131–155 (1, 3); OvW, 196 ± 21, 174–216 (1, 3); oAL, 43 ± 4, 36–51 (1, 11); oAW, 28 ± 5, 20–37 (1, 11).
Description: Colony encrusting, unilaminar, multiserial. Zooecia hexagonal to polygonal, separated by distinct grooves that may be altered by secondary calcification during ontogeny. Frontal wall slightly convex, initially even and smooth, later with gentle irregular ridges and/or umbos, usually associated with four small pseudopores; between three and five large, conspicuous, marginal areolar pores; ooecial pore small, oval, encircled by a thick rim of calcification. Primary orifice as long as is wide; anter two-thirds of a full circle, proximal margins very short, passing into rounded shoulders of a shallow but broad and widely U-shaped sinus, occupying about four-fifths of the total width, condyles short and blunt, comparatively small. A low peristome develops during ontogeny, formed by a raised ridge of secondary calcification of mother zooid and distal zooid, steeply sloping towards orifice distally, and extending proximolaterally, incorporating the suboral avicularium.
Ooecium initially globular, frontal being reduced to a semicircular outline during later ontogeny, when marginally covered by secondary calcification, ectooecium a broad smooth band encircling a rather flat, semicircular, frontal area of uncovered entooecium, marked by between six and ten prominent radiating ribs that thicken towards the margin; aperture orbicular.
Adventitious avicularia single, small, oval, located proximolateral to orifice, situated on a slightly raised cystid that becomes incorporated into peristome during ontogeny, frontal plane at an acute angle to colony surface; rostrum semi-elliptical, directing proximolaterally or laterally, proximal uncalcified area semicircular; crossbar complete, without columella. Larger frontal avicularia were not observed.
Remarks: Arístegui Ruiz (1984: 373, fig. 80b, c; pl. 31, figs 1–3) originally introduced this species as Lagenipora macroavicularia in his PhD thesis, and only three years later, using the same material and photos without referring to his previous account, erected B. porcellanum ( Arístegui Ruiz, 1987) . However, as the thesis was never officially published, and this species has never been cited thereafter, the name macroavicularia is suppressed here for porcellanum .
This species is characterized by its uneven frontal wall that develops during ontogeny, its large and round areolar pores, its asymmetrical peristome, the proximolateral position of the suboral avicularium, the absence of frontal avicularia, and by the few but prominent ribs on its small exposed entooecial area. Although not directly observed, we assume that, similar to B. muriella sp. nov. and Buffonellaria sp. 1 ( Fig. 1 View Figure 1 ), pseudopores are formed during early development of the frontal wall of the primary skeleton. Together with Buffonellaria sp. 2 (see below), B. porcellanum is one of the species that retain small but relatively conspicuous pseudopores, even in later ontogeny.
Buffonellaria porcellanum is recorded from depths of 3–31 m on rocks, shells, and other calcareous substrates. It has not been reported from areas other than the Canary Islands.
( FIG. 15A, B View Figure 15 )
Material examined: SMF 3015 About SMF , Sierra Leone , RV ‘Meteor’ cruise 1973, Stn 196 ( 7°27′N, 13°7′W), 81 m, on shell GoogleMaps .
Measurements: ZL, 445 ± 28, 384–489 (1, 20); ZW, 376 ± 41, 294–464 (1, 20); OL, 101 ± 6, 92–113 (1, 13); OW, 92 ± 4, 83–96 (1, 13); oAL, 43 ± 3, 37–47 (1, 14); oAW, 28 ± 2, 25–31 (1, 14); fAL, 100 ± 7, 88–108 (1, 7); fAW, 44 ± 3, 40–47 (1, 7).
Description: Colony encrusting, unilaminar, multiserial. Zooecia hexagonal, separated by shallow grooves. Frontal wall slightly convex, surface rough with several pustules developing during ontogeny, each pierced by a tiny pseudopore, and between three and six oval or slit-like, marginal, areolar pores. Primary orifice slightly longer than wide; anter horseshoeshaped, proximal margins very short and sloping, passing into a deep, broad, and widely U-shaped or rounded V-shaped sinus, occupying about two-thirds of the total width, condyles conspicuous, short but broad, blunt.
Ovicells were not observed.
Small oral avicularia, single, situated proximolateral to orifice, close to the zooid margin, oval, situated on a slightly raised cystid; rostrum semi-elliptical, directing laterally or proximolaterally; crossbar complete, without columella but slightly curved, proximal uncalcified area therefore sickle-shaped. Additional larger avicularia occasionally present, also in ontogenetically young zooids, situated opposite to small avicularium, albeit slightly more proximally, on a relatively small imperforate cystid; rostrum elongate triangular, slightly acute to frontal plane, narrowing distal to crossbar, and becoming very slender and parallel-sided, pointing distally or distolaterally; proximal uncalcified area semicircular, distal area elongate triangular; crossbar complete, without columella.
Remarks: This taxon differs from the species described above in having a pustulose frontal surface, conspicuously large and rounded condyles, a small single avicularium situated lateral or proximolateral to the orifice, and in having a larger frontal avicularium, with a long and thin rostrum directing distally or distolaterally, located proximolaterally to the orifice. However, because only a single colony lacking ovicells was available for study, this species is kept in open nomenclature. The frontal wall in Buffonellaria sp. 2 retains small pseudopores throughout its ontogeny, penetrating the small pustules.
Several zooecia in this colony display regeneration features, some of which have an intact skeleton, but a second or even third orifice implanted in the primary one. This suggests that these zooids were fed upon by a partial predator, possibly a nudibranch gastropod boring through the operculum and sucking out the internal organs without damaging the skeleton (e.g. McKinney, Taylor & Lidgard, 2003), after which a new zooid – an intramural bud (see Taylor, 1988b) – was produced within the older one.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Buffonellaria
| Berning, Björn & Kuklinski, Piotr 2008 |
Buffonellaria divergens ( Smitt, 1873 )
| Hayward PJ & Ryland JS 1999: 356 |
| Hayward PJ & Ryland JS 1979: 204 |