Creophilus erythrocephalus, (FABRICIUS)
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2011.00725.x |
|
publication LSID |
lsid:zoobank.org:pub:FBFE9195-BE04-4AFE-9417-6E38BCE6AB84 |
|
persistent identifier |
https://treatment.plazi.org/id/039B414F-1944-FFC0-FF49-FD214B35FD87 |
|
treatment provided by |
Valdenar |
|
scientific name |
Creophilus erythrocephalus |
| status |
|
6. CREOPHILUS ERYTHROCEPHALUS (FABRICIUS) View in CoL
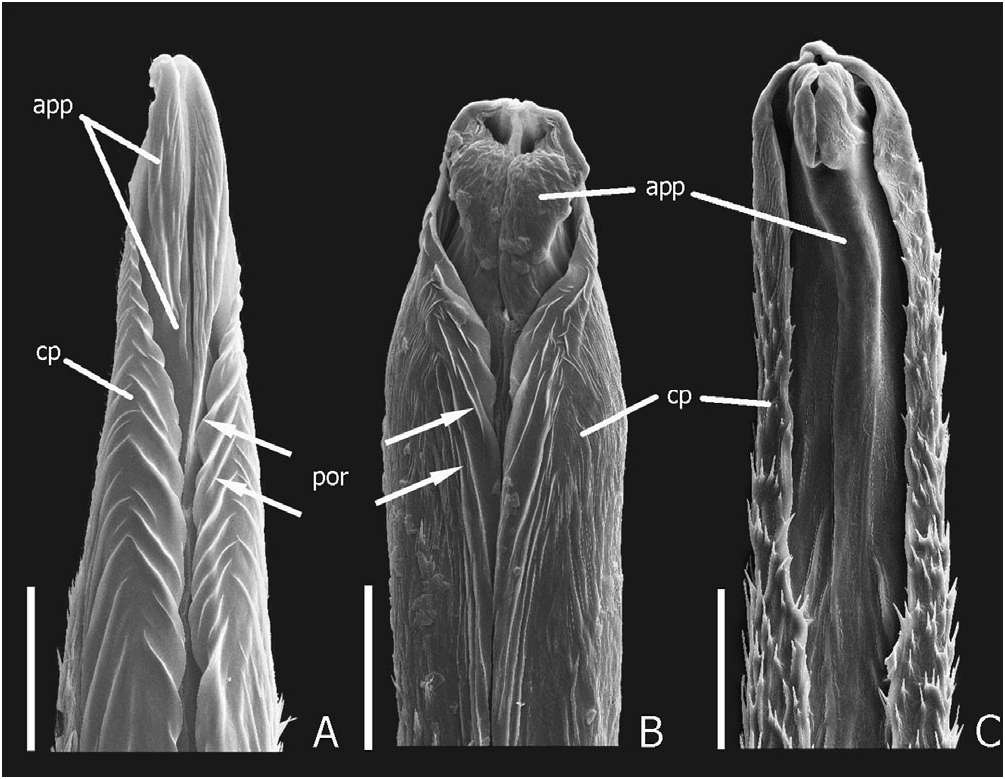
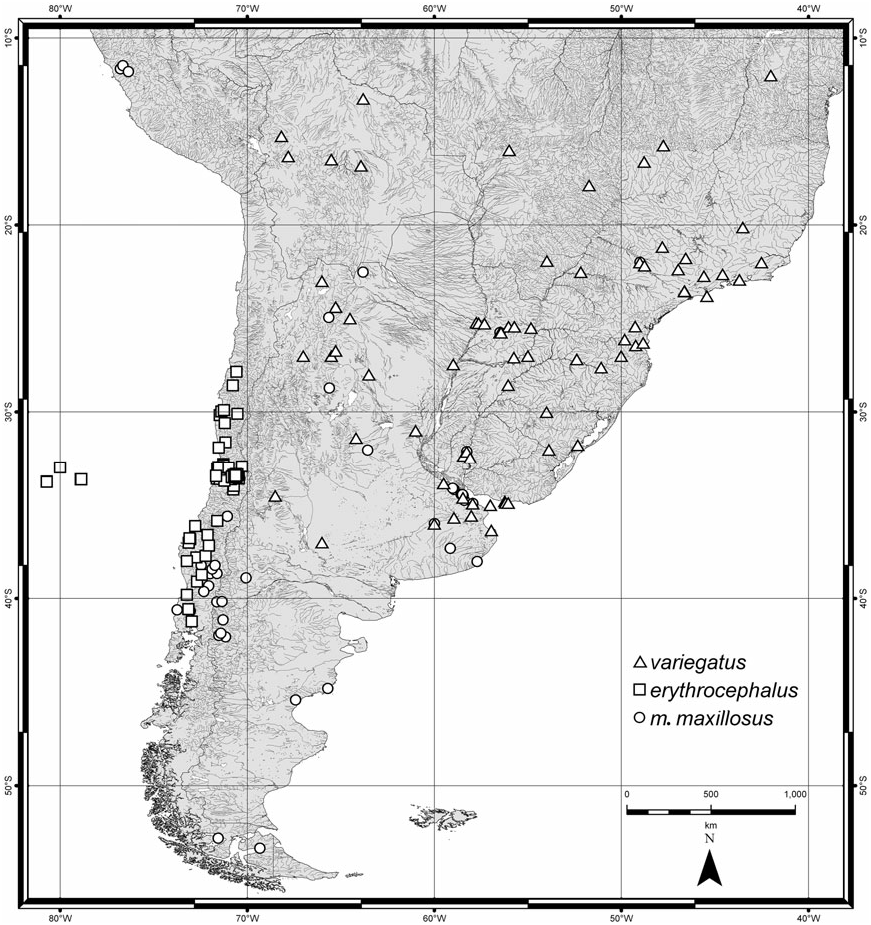
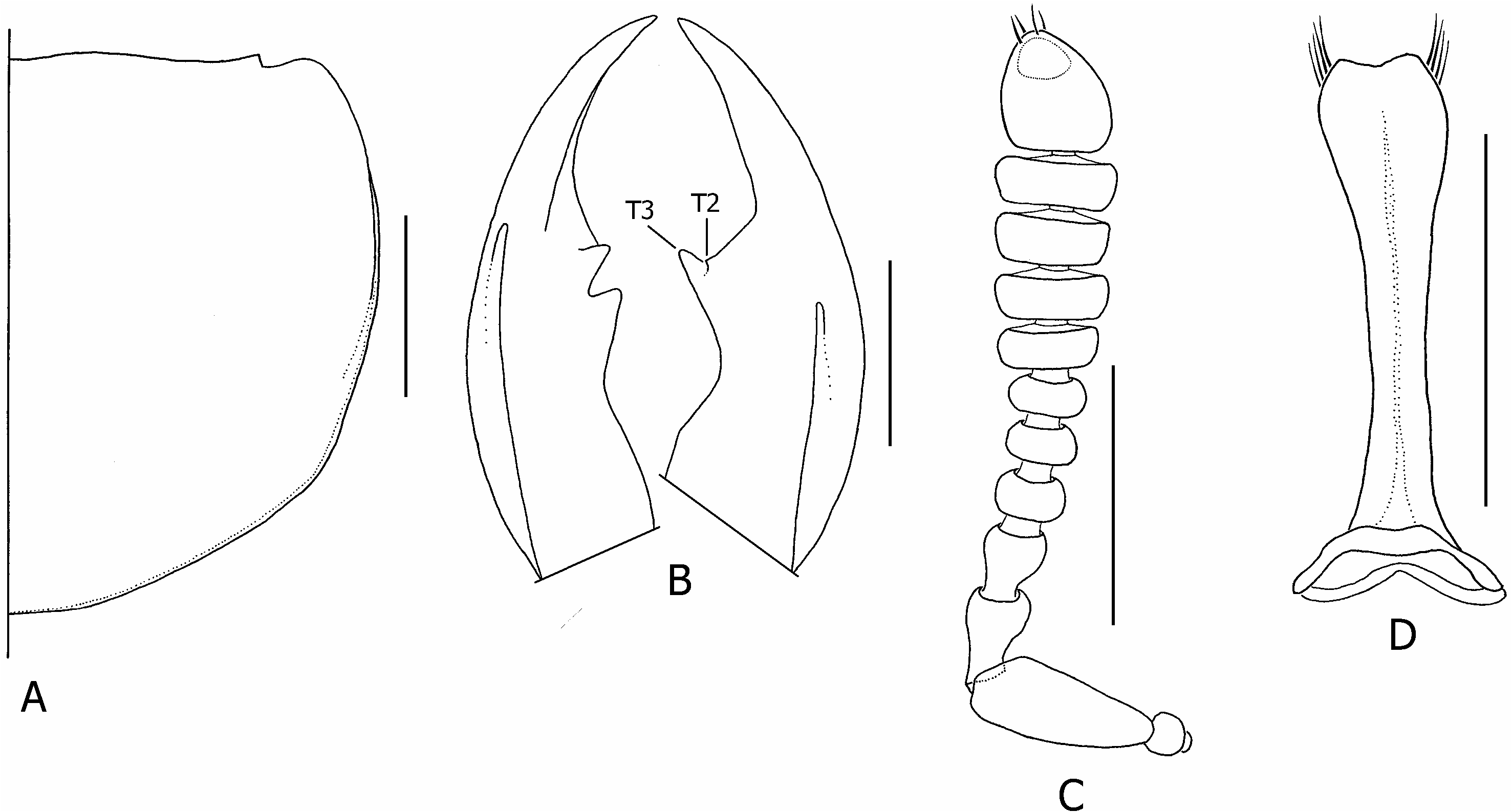
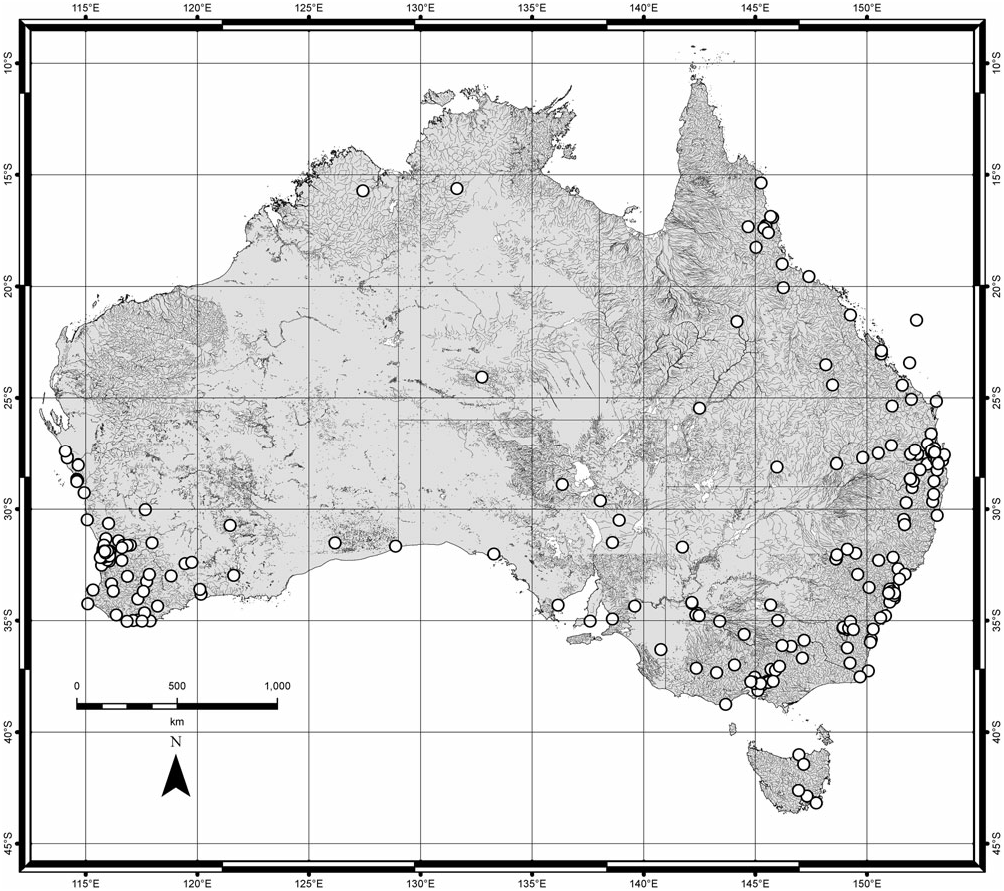
( FIGS 1A, 2A, B, L, 3A, B, G, H, O, 4H View Figure 4 , 5B View Figure 5 , 7A View Figure 7 , 10C View Figure 10 , 24 View Figure 24 , 25 View Figure 25 , 26 View Figure 26 , 28 View Figure 28 )
Staphylinus erythrocephalus Fabricius, 1775: 265 View in CoL . Type locality: ‘nova Hollandia’; Goeze, 1777: 724; Fabricius, 1781: 335 (not seen); Fabricius, 1787: 220; Fabricius, 1793: 523; Olivier, 1795: (42): 12, pl.2, fig. 9; Fabricius, 1801: 593; Turton, 1802: 511; Latreille, 1804: 293; Gravenhorst, 1806: 127; Dejean, 1821: 21; Boisduval, 1835: 55; Erichson, 1839: 351; Blanchard, 1842: 77; Fairmaire, 1849: 288; Murray, 1870: 57, 89; Steel, 1949: 57, 58 (synonym of C. lanio View in CoL ‘ex parte’); Zimsen, 1964: 231; Radford, 1981: 174 (invalid holotype designation).
Staphylinus unipunctatus Hope, 1831: 32 View in CoL . Type locality: Nepal; Lea, 1925: 229 (synonym of C. erythrocephalus View in CoL ); Steel, 1949: 57 (synonym of C. erythrocephalus View in CoL ); Radford, 1981: 174 (as C. punctatus , error for unipunctatus View in CoL ); Herman, 2001b: 3316 (synonym of C. erythrocephalus View in CoL ).
Emus erythrocephalus ; Dejean, 1833: 59 (not seen); Fauvel, 1877: 249; Fauvel, 1878a: 248; Fauvel, 1878b: 541; Fauvel, 1889: 261.
Creophilus erythrocephalus View in CoL ; Nordmann, 1837: 23; Motschulsky, 1858a: 49; Fauvel, 1875: 56; Olliff, 1887: 492; Olliff, 1889: 78, 81; Fauvel, 1903: 267; Froggatt, 1907: 137; Bernhauer, 1908: 19; Sharp & Muir, 1912: 498; Bernhauer & Schubert, 1914: 398; Bernhauer, 1920: 16; Fullaway, 1923: 185; Swezey, 1923: 303; Cameron, 1924: 87 (probable error for C. imitator View in CoL ; specimen not seen); Lea, 1925: 229; Tillyard, 1926: 209; Cameron, 1933: 83; Fuller, 1934: 14, 18; Cameron, 1937: 104 (error for C. imitator View in CoL ; see Distribution section, below); Hawkins, 1942: 885; Curran, 1945: 174; MacKeown, 1944: 127; Steel, 1949: 57, 59, 60, 61, figs 1, 4, and 9; Gourlay, 1950: 186; Bornemissza, 1957: 6; Coiffait & Sáiz, 1968: 364, figs 3 and 4; Sáiz, 1971: 349 (as erytrocephalus); Meyer-Rochow, 1972a, b, 1974; Bellas, Brown & Moore, 1974; Coiffait, 1976: 218; Legner, 1978: 348, 352; Radford, 1981: 174; Frank, 1982: 34; Matthews, 1982: 7, fig. 34 (cover picture); Andrews & Gibbs, 1989: 107, 113; Sáiz, Solervicens & Ojeda, 1990: 50, fig. 16 (as erytrocephalus); Kukalová-Peck & Lawrence, 1993: 236, fig. 46; Naumann, 1993: 163; (common name as devil’s coachhorse) Lawrence & Britton, 1994: 11, 97, fig. 5f, pl. 10c; Nishida, 1994: 71; Desender & Baert, 1996: 43; Newton, 1997: 25; Smithers, 1998: 16; Herman, 2001b: 3316; Levot, 2003: 32; Archer, 2004; 35; Nowak, 2004: 13; Read & Wilson, 2004: 54; Hawkeswood & Turner, 2008.
Type material: Staphylinus erythrocephalus Fabricius. Lectotype (designated in footnote by Steel, 1949: 58, labelled by me). ♀, ‘Staph. erythroceph.| Fab. Entom.p.265.6 / Creophilus | erythrocephalus| F.| det. A. F. Newton 1989/ FMNH-INS 0000 016 779/ [red] LECTOTYPE | Staphylinus | erythrocephalus Fabricius, 1775 | des. by W. O. Steel, 1949, teste | D. J. Clarke 2008’ (in Banks Collection, BMNH). Specimen glued to pin ventrally, wings unfolded, missing left antennomeres 3–11, right prothoracic leg missing tibia and tarsus, but coxa and femur attached to specimen with glue. Paralectotypes (2). 1♀, no data but with labels ‘ Creophilus | lanio Er. | det. A. F. Newton 1989/ FMNH-INS 0000 016 780/ [yellow] PARALECTOTYPE | Staphylinus | erythrocephalus Fabricius, 1775 | des. by W. O. Steel, 1949, teste | D. J. Clarke 2008/ Creophilus lanio ( Erichson, 1839) det. D. J. Clarke 2006’ (in Banks Collection, BMNH). Specimen pinned through base of abdomen, glued to pin ventrally, missing right metathoracic leg, and with wings unfolded but partly disintegrated. 1♂, no data but with labels ‘[yellow] PARALECTOTYPE | Staphylinus | erythrocephalus Fabricius, 1775 | des. by W. O. Steel, 1949, teste | D. J. Clarke 2010/ Creophilus lanio ( Erichson, 1839) det. D. J. Clarke 2010’ (in ZMUC). Specimen pinned in unit tray with label pinned in tray: ‘erythro| cephalus’ [Fabricius’s handwriting]. The lectotype and female paralectotype are pinned with thick pins typical of the period ( Andrews & Gibbs, 1989). The male paralectotype ( ZMUC) has a hole in the left elytron consistent with the size of the lectotype pin, but was at some point re-pinned with a modern pin through the right elytron. Steel’s (1949) lectotype designation is valid under Article 74.5 ( ICZN, 1999). Both paralectotypes are specimens of C. lanio .
Staphylinus unipunctatus Hope. View in CoL Lectotype (here designated). ♀, ‘[circular with red border] Type| H.T./ unipunctatus. Hope/ Hardwicke | Bequest./ unipunctatus. Hope.| 4242 [in pencil] erythrocephalus Fabr. Hopes View in CoL locality wrong/ FMNH-INS 0000 016 783/ [red] LECTOTYPE | Staphylinus View in CoL | unipunctatus Hope, 1831 View in CoL | designated by| D.J. Clarke 2008’ (in BMNH). Hope did not mention the number of specimens examined. Although only a single specimen was in Hardwicke’s collection, there is insufficient evidence to consider it the holotype, as labelled.
Other material examined: 1513 specimens. See supporting information, Appendix S 1.
Diagnosis: With characters of the erythrocephalus - group; head orange-red, with transverse diameter of circular medial black spot greater than 0.5 ¥ EYL ( Figs 1A, 7A View Figure 7 ); right mandible with two distinct teeth ( Fig. 25B View Figure 25 ); elytra metallic blue, humeri glabrous, shining black; abdomen uniformly black; tergal chaetotaxic formula = 4-6-6–6(4)-4-6.
Description: Measurements ( N = 10♂, 10♀). Forebody length: ♂ 5.1–9.4 mm, ♀ 5.3–7.8 mm. See supporting Table S 5 for comparison of ranges of male and female ratios. Head. Head ( Figs 2A, B, 7A View Figure 7 ) orange/red, narrowly black around mouthparts and antennal fossae, vertex with large circular and sharply defined black spot, situated posterior to and not concealing dorsal tentorial pits, with transverse diameter greater than 0.5¥ EYL; strongly trapezoidal, much wider posteriorly; HW/ HL = 1.37–1.69; shining, without distinct microsculpture; eyes small to moderately large ( EYL / HL = 0.37–0.53), dorsolateral; lateral margins of head visible in dorsal view (not obscured by eye); HL 1/ HL 2 greater in females than males (♂ = 1.38–1.83, ♀ = 1.50–2.20); antennae as in Figure 25C View Figure 25 , antennomeres 1–6 black, 7–11 greyish-black, 11 as long as or very slightly longer than 9–10 together; mandibles as in Figure 25B View Figure 25 , moderately longer than head in large males, subequal to head in females ( ML / HL ♂ = 0.84– 1.24, ♀ = 0.81–1.00), right mandible with two teeth, T 1 absent, T 3 much larger than T 2; left mandible with ventral basolateral ridge only weakly developed. Thorax and abdomen. Pronotum ( Fig. 25A View Figure 25 ) slightly transverse ( PW / PL = 1.10–1.19); PL 1.24–1.57 ¥ ESL; with basolateral margins very slightly emarginate, hind angles indistinct; with short and sparse peripheral vestiture, and dense longer vestiture on anterolateral declivities; elytra, except humeri, metallic blue or violet, densely setose, and only very slightly rugose; humeral regions black, shining, distinctly callused; wings fully developed, black with distinct black spot in medial field between MP 3 and MP 4 veins; abdomen shining and uniformly black; tergite VII with welldeveloped palisade fringe. Male genitalia and secondary sexual characters. Sternite VII with impressed transverse region of increased setal density, containing high density of micropunctures. Aedeagus as in Figure 3A, B; median lobe apex produced distinctly dorsad into short and broad globular process ( Fig. 3O). Paramere as in Figure 25D View Figure 25 . Internal sac inverted as in Figure 3A and B, everted as in Figure 3H; paired apicolateral sclerites (as) separated from sclerotized median lobe by distinct membranous strip; ventral sclerite of internal sac flattened ( Fig. 3G), without conspicuous sculpture. Female internal genitalia. Internal female genitalia as in Figure 5B View Figure 5 ; vaginal plate with paired lateral sclerites (pls), posterolateral aspects membranous; vaginal fold (vf) with pair of separated sclerites dorsal to posterolateral lobes of vaginal plate. Chaetotaxy. Elytral discal series with three macrosetae; metaventrital macroseta present; tergal chaetotaxic formula = 4-6-6-6(4)-4-6, medial pair of macrosetae absent on tergite III, inner lateral macrosetae present or absent on tergite VI, absent on tergite VII.
Comparison: Creophilus erythrocephalus is most similar to C. imitator and C. lanio , but the metallic blue elytra with shining black humeri and large, distinctly circular black cranial spot are diagnostic. In addition, C. erythrocephalus may be easily distinguished from C. lanio by its black ninth abdominal segment.
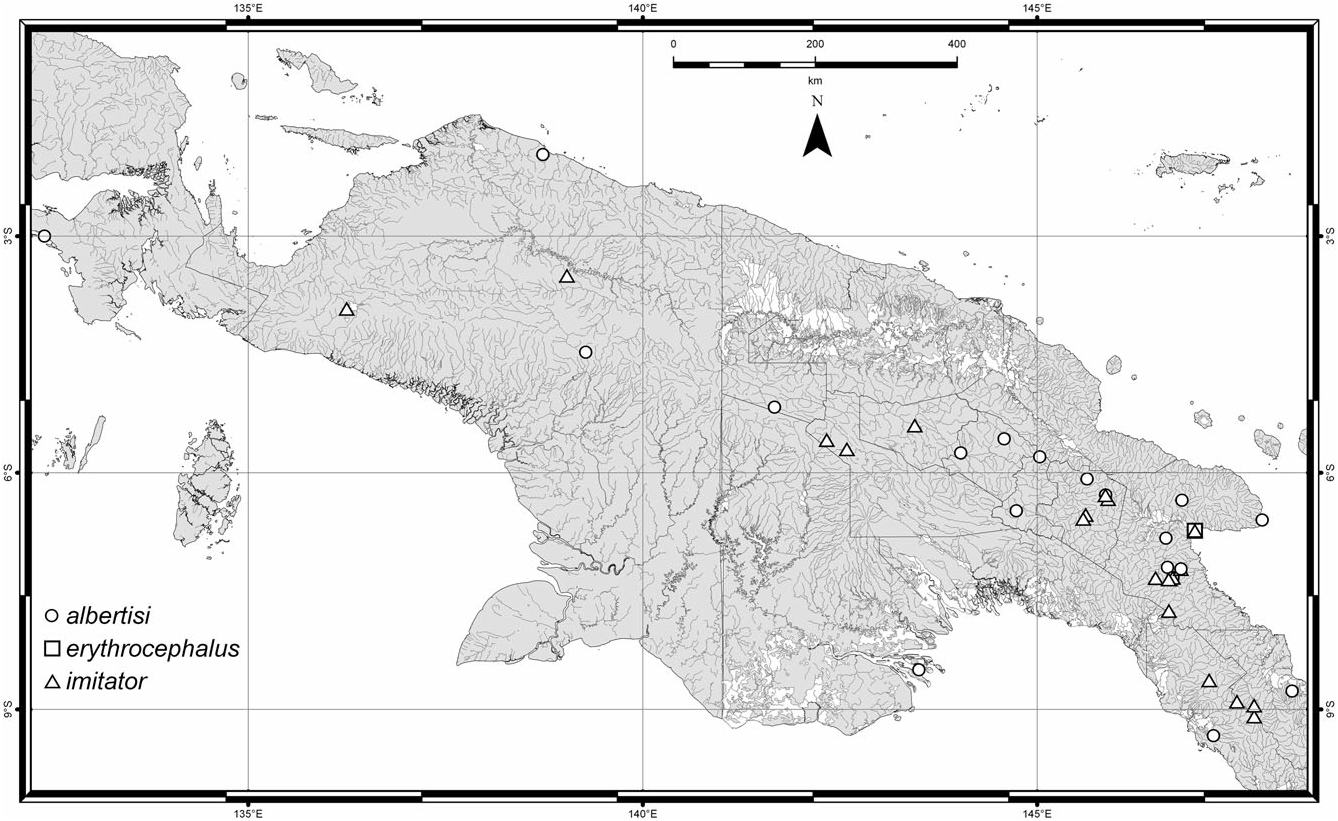
Distribution ( Figs 24 View Figure 24 , 26 View Figure 26 , 28 View Figure 28 ): Herman (2001b) recorded C. erythrocephalus from Nepal, New Guinea, Australia, Lord Howe Island, New Caledonia, Tonga, Society Islands ( French Polynesia), Hawaii, Easter Island, and Chile. It is apparently native to Australia and introduced to Chile ( Fauvel, 1903) where it is well established [earliest records from 1898 ( Sáiz, 1971), in MNNC]. It was deliberately introduced to Hawaii ( Fullaway, 1923; Swezey, 1923) but never established there ( Newton, 1997). Old New Zealand records are doubtful (e.g. Fairmaire, 1849; Murray, 1870; Curran, 1945; three old specimens labelled ‘Neuseeland’) and it is certainly absent from there at present. The Nepal locality for the S. unipunctatus lectotype is almost certainly incorrect (see label data quoted above); no other Nepalese records were seen. It also occurs in Fiji (five specimens, Taveuni Island). Historical New Guinean records are probably mislabelled: a single specimen (‘1 example. Doormanpadbivak 1410 m. Oct. 1920 ’) collected by W. C. van Heurn during his 1920–21 expedition to Dutch North New Guinea was recorded as C. erythrocephalus by Cameron (1924) (probably in Zoological Museum, Amsterdam). Later, Cameron (1937) listed another New Guinean specimen (Mt Tafa, alt. 8500 ft., ii.1934; FMNH-INS 0000 016 773, in BMNH) that I determined to be C. imitator . I have seen one recent specimen from New Guinea (‘Lae, TPNG, VIII 59, A. C. Robinson, UQIC Reg.#80897) and there is nothing to suggest it is mislabelled ( TPNG = Territory of Papua New Guinea).
Biology and ecology: Creophilus erythrocephalus prefers open or disturbed habitats, and typically is found in synanthropic situations. Many specimens have been taken at light ( UV, blacklight), dung, and carrion of various sorts. Habitat: Banksia and Eucalyptus forest/scrub; sclerophyll forest and woodland, coastal sand heath, sand dunes, and beach. Altitude: sea level to 1600 m. Phenology: throughout the year. Larvae and pupae are known and will be described elsewhere. Creophilus erythrocephalus is a known predator of fly larvae, and has been tested as a biocontrol agent for flies in Hawaii ( Fullaway, 1923; Swezey, 1923). Dynamics of arrival and departure at carrion have been recorded in Australia ( Fuller, 1934; Bornemissza, 1957; Levot, 2003; Archer, 2004). Results of these studies are consistent with those discussed under C. maxillosus , above. Fuller (1934) noted that larvae, while developing, can remain at a carcass for periods exceeding 20 days and that, in agreement with other studies, adults tend to occur at a carcass only intermittently.
Remarks: Fabricius did not indicate the number of specimens examined, or designate a ‘type’. Zimsen (1964: 231) listed two type specimens for Staphylinus erythrocephalus , one in Kiel, one in London ( BMNH: Banks Collection). The former specimen was transferred to ZMUC, along with the rest of Fabricius’ collection, and the existence of a type in his personal collection is reasonable as ‘Banks was a generous man... Fabricius certainly added specimens from the collections he studied to his own collection’ ( Radford, 1981: 157). C. O. Waterhouse studied the Banks Collection ( BMNH) in the late 19 th century, and, according to Radford (1981: 156), he applied type labels to specimens he was reasonably sure were those to which Fabricius’s descriptions referred. There are, however, two specimens in the Banks Collection ( BMNH), and based on their aforementioned details, both of these and the ZMUC specimen can be inferred to have been studied by Fabricius and should be considered syntypes. It is possible that Zimsen did not list the second BMNH specimen because Steel (1949) previously identified it as C. lanio . Radford (1981) also studied the Fabricius type material, but considered the now lectotype specimen to be a holotype, probably basing this on the original description, C. O. Waterhouse’s presumably attached ‘type’ label, and by exclusion of the C. lanio specimen. According to Radford (1981: 174), the lectotype should have had a second label with ‘ BMNH Staphylinus erythrocephalus’, and a museum (Waterhouse’s?) type label. No such labels were present on either specimen although the type label pinned in the drawer next to the specimens could have once been applied to the lectotype.
| F |
Field Museum of Natural History, Botany Department |
| A |
Harvard University - Arnold Arboretum |
| W |
Naturhistorisches Museum Wien |
| O |
Botanical Museum - University of Oslo |
| J |
University of the Witwatersrand |
| ZMUC |
Zoological Museum, University of Copenhagen |
| S |
Department of Botany, Swedish Museum of Natural History |
| N |
Nanjing University |
| HL |
Houghton Lake Wildlife Research Station |
| ML |
Musee de Lectoure |
| T |
Tavera, Department of Geology and Geophysics |
| PW |
Paleontological Collections |
| PL |
Západoceské muzeum v Plzni |
| MP |
Mohonk Preserve, Inc. |
| VI |
Mykotektet, National Veterinary Institute |
| MNNC |
Museo Nacional de Historia Natural, Santiago |
| C |
University of Copenhagen |
| I |
"Alexandru Ioan Cuza" University |
| TPNG |
Department of Primary Industry |
| UQIC |
University of Queensland Insect Collection |
| UV |
Departamento de Biologia de la Universidad del Valle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Creophilus erythrocephalus
| Clarke, Dave J. 2011 |
Creophilus erythrocephalus
| Nowak R 2004: 13 |
| Read JL & Wilson D 2004: 54 |
| Levot GW 2003: 32 |
| Herman LH 2001: 3316 |
| Smithers CN 1998: 16 |
| Newton AF 1997: 25 |
| Desender K & Baert L 1996: 43 |
| Lawrence JF & Britton EB 1994: 11 |
| Nishida GM 1994: 71 |
| Kukalova-Peck J & Lawrence JF 1993: 236 |
| Naumann ID 1993: 163 |
| Saiz F & Solervicens J & Ojeda P 1990: 50 |
| Andrews JRH & Gibbs GW 1989: 107 |
| Frank JH 1982: 34 |
| Matthews EG 1982: 7 |
| Radford WPK 1981: 174 |
| Legner EF 1978: 348 |
| Coiffait H 1976: 218 |
| Saiz F 1971: 349 |
| Coiffait H & Saiz F 1968: 364 |
| Bornemissza GF 1957: 6 |
| Gourlay ES 1950: 186 |
| Steel WO 1949: 57 |
| Curran CH 1945: 174 |
| MacKeown KC 1944: 127 |
| Hawkins CN 1942: 885 |
| Cameron M 1937: 104 |
| Fuller ME 1934: 14 |
| Cameron M 1933: 83 |
| Tillyard RJ 1926: 209 |
| Lea AM 1925: 229 |
| Cameron M 1924: 87 |
| Fullaway DT 1923: 185 |
| Swezey OH 1923: 303 |
| Bernhauer M 1920: 16 |
| Bernhauer M & Schubert K 1914: 398 |
| Sharp D & Muir F 1912: 498 |
| Bernhauer M 1908: 19 |
| Froggatt WW 1907: 137 |
| Fauvel A 1903: 267 |
| Olliff AS 1889: 78 |
| Olliff AS 1887: 492 |
| Fauvel A 1875: 56 |
| Motschulsky V 1858: 49 |
| Nordmann A 1837: 23 |
Emus erythrocephalus
| Fauvel A 1889: 261 |
| Fauvel A 1878: 248 |
| Fauvel A 1878: 541 |
| Fauvel A 1877: 249 |
| Dejean PFMA 1833: 59 |
Staphylinus unipunctatus
| Herman LH 2001: 3316 |
| Radford WPK 1981: 174 |
| Steel WO 1949: 57 |
| Lea AM 1925: 229 |
| Hope FW 1831: 32 |
Staphylinus erythrocephalus
| Radford WPK 1981: 174 |
| Zimsen E 1964: 231 |
| Steel WO 1949: 57 |
| Murray A 1870: 57 |
| Fairmaire L 1849: 288 |
| Blanchard E 1842: 77 |
| Erichson WF 1839: 351 |
| Boisduval JBA 1835: 55 |
| Dejean PFMA 1821: 21 |
| Gravenhorst JLC 1806: 127 |
| Latreille PA 1804: 293 |
| Turton W 1802: 511 |
| Fabricius JC 1801: 593 |
| Fabricius JC 1793: 523 |
| Fabricius JC 1787: 220 |
| Fabricius JC 1781: 335 |
| Goeze JAE 1777: 724 |
| Fabricius JC 1775: 265 |