Mesomphaliini Hope 1840
|
publication ID |
https://doi.org/ 10.1080/00222933.2014.909060 |
|
persistent identifier |
https://treatment.plazi.org/id/039A87D5-FFC9-FFFF-C37B-61153C66FE7D |
|
treatment provided by |
Felipe |
|
scientific name |
Mesomphaliini Hope 1840 |
| status |
|
Tribe Mesomphaliini Hope 1840 View in CoL (16 genera, ~550 species)
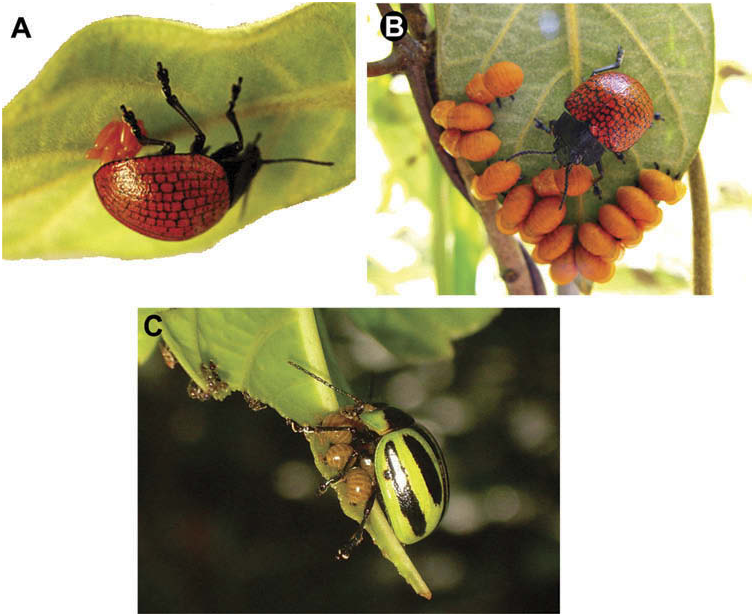
Chaboo (2001) provided a phylogenetic revision of the three known species of Acromis Chevrolat. Author Westerduijn has discovered a new subsocial species in Peru. Flavia Fernandes (pers. comm.) is describing two new subsocial species from Brazil. Natural history is documented for Acromis sparsa Boheman in Costa Rica and Panama ( Windsor 1987; Trillo 2008; CSC unpublished data) and for Acromis spinifex (Linnaeus) in Brazil ( Buzzi 1988) and Peru, and Trinidad and Tobago (CSC unpublished data). These species use convolvulaceous vines as hosts and exhibit oviparity, gregarious larvae capable of cycloalexy, faecal shields in larvae and pupae, extreme adult sexual dimorphism, aggressive maternal guarding, active maintenance of larval broods, and maternal guidance to new leaves. Male polymorphism ( Chaboo 2001) and male fighting ( Windsor 1987; Bailey and Ridsdill-Smith 1991; Trillo 2008) are known in A. sparsa . Egg masses range from 30– 44 eggs in A. sparsa and 15 in A. spinifex ( Buzzi 1988; Chaboo 2001). Males can court mothers with offspring ( Figure 1D View Figure 1 ). Acromis sparsa females have been observed fighting other mothers to adopt their offspring ( Trillo 2008) and amalgamation of broods, particularly late larval instars is fairly common (>40% of broods). We report below three new records of subsocial Acromis species from Brazil, including the two new species.
Acromis venosa Erichson 1847 , New Record ( Figure 1E View Figure 1 ; Table 1). RW observed multiple populations of A. venosa in Peru: Cuzco Province, San Pedro, Cock of the Rock Lodge, 71°32’44.6”W 13°03’21.8”S, 1400 m, road through foothill forest, I.2005. Many adults were found feeding on a single extensive vine of Convolvulus sp. (Convolvulaceae) , growing along the road. At this same site on 6.XII.2009, many adults were observed on a similar plant, including two females guarding pendant egg clutches (12 and 24 eggs each); one of these females was mating while guarding. A third female was guarding larvae that were arranged in a cycloalexic formation. This is the first host plant record for this species.
RW has collected a new Acromis species with maternal care in Peru. Two new Brazilian species are being described (Flavia Fernandes, personal commun.); one was observed with maternal care in Caceres, Mato Grosso, Brazil, and the other in Corumbá, Mato Grosso do Sul, Brazil (FFC). One of these new Brazilian species may be the same as the new Peruvian species.
Acromis new species 1, New Record ( Table 1). This putative new species was observed twice by RW at two different localities. Site 1: Peru: San Martin Prov.: Tarapoto, 6°30’32.00” S, 76°22’24.00” W, elev. 243.84 m, 5.I.2005; two females were guarding broods, one with ~ 19 eggs and an instar I larva and the other with ~ 19 eggs. They occupied a vine, Ipomoea umbellata (L.) H. Hallier ( Convolvulaceae ), which was growing along a stream. Adult feeding damage on the plant was observed. Site 2: Peru: San Martin Prov.: Juan Guerra, 6°35’00.16” S, 76°19’54.80” W, elev. 204.22 m, 12.V.2008; one female was with four instar III larvae, on Ipomoea L. sp. ( Convolvulaceae ). Flavia Fernandes (pers. commun.) believes she has two new Acromis species , both subsocial, in Brazil (New Records, Table 1); it is possible that one of her new species is the same as the new Peru species. We await the Fernandes’ study for further information.
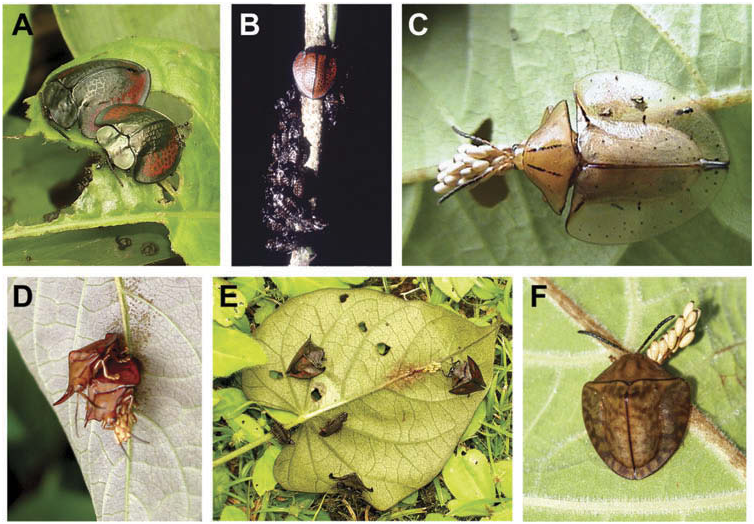
Omaspides Chevrolat View in CoL comprises three subgenera and 40 species ( Borowiec 2010; Borowiec and Świętojańska 2012). Subsociality occurs in all eight species whose immature stages are known ( Figures 2–4 View Figure 2 ; Table 1). Egg masses resemble those of Acromis View in CoL , comprising 28– 80 eggs, arranged in a pendant grape-like cluster; females attend offspring for over two months. Buzzi (1988) reported an aggregation of> 100 pupae, which suggests that larval groups may be larger. Larvae possess shields and form cycloalexic rings. Sexual dimorphism in Cassidinae is usually subtle but Omaspides (Paromaspides) haematidea (Boheman) View in CoL has males with angulate humeral extensions while females are more rounded (see Borowiec and Świętojańska 2012). Ipomoea View in CoL has been reported as the host of five species, including the known subsocial species ( Windsor et al. 1992; Rodriguez 1994a), and Theobroma (Sterculiaceae) View in CoL has been reported as host for one species ( Monte 1955). Below we report three new records of subsociality.
Omaspides (Omaspides) abbreviata Baly 1872 View in CoL , New Record ( Figure 2A – B View Figure 2 ; Table 1). Subsociality in this species was observed on several plants of Ipomoea sp. at a single site in Peru: San Martin Province, Aguas Claras, 1100 m, understorey of altered foothill forest, 14.II.2009. RW examined five broods – 49 instar II larvae but no guarding; female 1 with ~ 55 eggs, which form a pendant cluster; female 2 with 30 instar II larvae; female 3 with 54 instar II larvae; and female 4 with 38 instar II larvae. The unguarded group had likely lost its mother. Ipomoea View in CoL is the first host plant record for this species.
Omaspides (Omaspides) augusta Boheman 1856 , New Record ( Figure 2C – E View Figure 2 ; Table 1). RW observed subsociality many times on the convolvulaceous vine, Ipomoea philomega (Vell.) House in Peru: Loreto Prov., Picuroyacu, 150 m elev., a disturbed forest near stagnant water, 30.IV.2009. Observations included: one unguarded brood of 12 instar III larvae clustered on the underside of a leaf and a pair of adults mating on the upperside of the same leaf; five adults, 12 larvae, and one guarded cluster of 32 eggs on a single plant; and one unguarded brood of eight instar III larvae on the underside of a leaf. On 10.VI.2009, a female was found guarding a clutch of eggs ( Figure 2D View Figure 2 ); 11 days later, on 21.VI.2009, this female was still guarding the eggs, but some instar I had emerged. At a nearby forest border on 21.II.2010, a female was observed guarding a cluster of about 25 eggs on the underside of a leaf of the same Ipomoea species ; she actively defended her clutch. Ipomoea philomega is the first host plant record for the species. Doug Yanega (pers. comm.) observed one subsocial Omaspides species , possibly new, in Brazil and we recognise this as a third new record. The specimens are loaned to CSC for study.
We provide supplementary information and illustrations for the following two Omaspides species which were previously described as subsocial. Omaspides (Omaspides) convexicollis Spaeth, 1909 ( Figure 3A – B, Table 1). Rodriguez (1994a, 143) reported finding six females guarding egg clutches of about 50 larvae, one female guarding 11 larvae, and one female guarding nine pupae in Costa Rica on I. philomega . Each egg clutch was suspended by a single thread from the underside of leaves. Eulophid wasps were reared from eggs .
Omaspides (Omaspides) pallidipennis Boheman, 1854 View in CoL ( Figure 3C; Table 1). Gomes et al. (2012) reported on this species’ biology based on long-term studies of populations at three sites in Brazil: Minas Gerais, campus, Universidade Federal de Lavras, Reserva Biologica Unilavras/Boqueirão, and Floresta Nacional de Passa Quatro, 26. I.2007 –present, on Ipomoea saopaulista View in CoL O’ Donell. Adults appear on host plants in October, begin oviposition in December, and stop oviposition in May. Egg clusters are naked, lacking any coverings, and comprise on average 67.5 eggs; eggs hatch after about 2 weeks. Females care for their offspring for about 2 months, defending them from ants and hemipteran bugs that attack eggs and larvae. Larvae take about 29 days to reach the pupal stage. In April, as temperature falls and leaves senesce, new offspring are less likely to reach adulthood. Mothers do not feed during the egg or pupal stages but feed with their larvae. They stay with teneral adult offspring for about 3 days. Even with maternal guarding, eggs and larvae are subject to intense predation by ants and hemipteran bugs, so maternal presence is essential for any offspring surviving. Like O. (O.) tricolorata ( Frieiro-Costa and Vasconcellos-Neto 2003) View in CoL , females adopt offspring of other conspecific females; these adoptees can be of any age and are guarded with the same zeal as their own offspring ( Figure 2F View Figure 2 ). Several wasp parasitoids and parasites ( Hymenoptera View in CoL ) were reared from this Omaspides View in CoL population and identified as Brachymeria Westwood sp. ( Chalcididae View in CoL ; probably a new species according to Marcelo Teixeira Tavares, pers. commun.), Conura (Spilochalcis) Spinola sp. ( Chalcididae View in CoL ), and Emmersonella Girault sp. ( Hymenoptera View in CoL : Eulophidae View in CoL ). Other insect enemies of Omaspides View in CoL are shown in Figure 4 View Figure 4 .
Paraselenis Spaeth View in CoL currently comprises three subgenera and 29 species found mostly in Brazil ( Borowiec and Świętojańska 2012). Five subsocial species are documented, all in one subgenus: Paraselenis (Spaethiechoma) decipiens (Boheman) View in CoL ( Figure 2E–F View Figure 2 , new record), Paraselenis (Spaethiechoma) dichroa (Germar) View in CoL (= Echoma dichroa Germar, Bondar in Monte 1932; Buzzi 1988), Paraselenis (Spaethiechoma) flava Linnaeus View in CoL (= Omoplata flava L., Weyenberg 1874; Jolivet 1988a; Montes and Raga 2010), Paraselenis (Spaethiechoma) solieri (Boheman) View in CoL ( Monte 1932; Buzzi 1988), Paraselenis (Spaethiechoma) tersa (Boheman) View in CoL ( Windsor et al. 1992; Windsor and Choe 1994). These were documented on convolvulaceous vines; P. (S.) flava is a recognised pest of sweet potato in Brazil ( Montes and Raga 2010). Immature stages are described for only Paraselenis (Paraselenis) axillaris Sahlberg View in CoL ( Donceel 1885; Buzzi 1988) and Paraselenis decipiens Monte View in CoL (as Echoma decipiens ; Maulik 1948) but subsociality was not reported in these. Subsocial Paraselenis View in CoL females guard clusters of ~ 20 eggs which are attached along the ventral leaf midrib ( Buzzi 1988); females also guard all larval stages and the aggregated pupae.
Paraselenis (Spaethiechoma) dichroa Germar View in CoL ( Figure 3D, Table 1). FFC observed maternal care in Brazil: Minas Gerais: Floresta Nacional de Passa Quatro, Passa Quatro, 22°33’ S, 44°57’ W, 17.IV.2003. The host plant was Ipomoea sp L. ( Convolvulaceae View in CoL ), a new host for this species ( Borowiec and Świętojańska 2012).
Paraselenis (Spaethiechoma) decipiens (Boheman) New Record ( Figure 3E–F; Table 1). FFC observed courtship and egg hatching in Brazil: Minas Gerais: Floresta Nacional de Passa Quatro, Passa Quatro, 22°33’ S, 44°57’ W, II.2005. Ipomoea sp L. ( Convolvulaceae ) is a new host plant record, but Convolvulus sp. was previously reported ( Maulik 1948). Figure 2 View Figure 2 shows a female guarding her own pupae, as well as adopted pupae of two other mothers who were brooding on the same vine. A parasitoid wasp was reared and deposited in the Instituto Biológico de Sao Paulo, Brazil.
Subfamily Chrysomelinae
Chrysomelinae (150 genera, ca. 3000 species) has the most diverse reproductive biology among chrysomelid subfamilies as species exhibit sexual dimorphism, male fighting, and prolonged mating ( Dickinson 1996), parthenogenesis ( Cox 1996), oviparity, ovoviviparity, and viviparity ( Bontems 1984), and solitary, presocial, and subsocial behaviours ( Kudo and Hasegawa 2004). Pupation may be arboreal or underground ( Takizawa 1976). Both adults and larvae exhibit repulsive host-derived and glandular chemical secretions ( Termonia et al. 2001). Below we report two new records of subsociality, bringing known records to seven species in five genera – Doryphora View in CoL , Gonioctena View in CoL , Platyphora , Proseicela , and Pterodunga .
Tribe Doryphorini , Subtribe Doryphorina
Doryphora Illiger View in CoL currently comprises nine species (Mauro Daccordi, pers. commun.), from Central and South America ( Seeno and Wilcox 1982). Prestonia species hosts ( Apocynaceae View in CoL ) have been documented for two Doryphora species ( Eberhard 1981) . In one Doryphora species in Colombia, rows of eggs are laid on the leaf surface; both adults and larvae are cannibalistic towards eggs. Hatching is not synchronous and early hatched larvae will eat unhatched eggs. Larvae live gregariously, in groups from 2 – 40 individuals; groups of mixed age larvae are not unusual, probably due to amalgamation or adoption. Pupation is solitary and underground. As adults, both males and females have mesosternal horns that the males use aggressively to lift and push other males. It is unknown how females use their horns.
Doryphora reticulata (Fabricius) New Record ( Figure 5A – B View Figure 5 ; Table 1). FFC has studied this species in two sites in Brazil: Reserva Biológica Unilavras, Boqueirão, 21° 20’47” S, 44°59’27” E, 2.V.2005 – 04.X.2005 and Floresta Nacional de Passa Quatro, Passa Quatro, 2.V.2011 – 4.IV.2011. Larval groups comprised about 14 individuals (n = 3) and were found aggregated in the mornings on the abaxial sides of leaves of Prestonia tomentosa R. Br. (Apocynaceae) View in CoL , with their mother over them. By mid-morning, larvae began walking away from the group, but the mother moved towards them and the group quickly reassembled into cycloalexic formations. When touched with a stick (four occasions), the female tried to defend the group, biting the stick with her mandibles. By late morning, larvae began feeding on the most apical leaves, and moved later in a single file to new lower leaves. Tachinid parasitoids ( Diptera View in CoL ) were reared from larvae.
Platyphora Gistel comprises ~500 species from tropical South America and the host plants are in Asteraceae View in CoL , Solanaceae View in CoL ( Jolivet and Hawkeswood 1995; Medeiros et al. 1996; Daccordi, pers. commun.), and Convolvulaceae View in CoL ( Costa Lima 1936). Members may exhibit viviparity, cycloalexic larvae, and chemically defended aposematic larvae and adults ( Plasman et al. 2001). Viviparity is known in Platyphora biforis (Germar) (= Platyphora anastomozans Perty ), Platyphora nigronotata (Stål) ( Medeiros and Vasconcellos-Neto, 1994) , Platyphora fasciatomaculata (Stål) , and Platyphora quadrisignata (Germar) ( Schroder et al. 1994; Medeiros et al. 1996). Female P. fasciatomaculata larviposit clusters of up to 30 larvae that are immediately capable of cycloalexic behaviour ( Medeiros et al. 1996). Four Platyphora species are known to be subsocial. A female of Platyphora conviva (Stål) was found guarding larvae on a Solanum View in CoL host ( Jolivet et al. 1990). Orange-black females of Platyphora microspina Bechyné guard small clusters (~4) of orange-coloured larvae ( Discover Life, 2011). A third species, Platyphora selvae Daccordi (= Labidomera suturella Chevrolat, Reid et al. 2009 ), was observed multiple times in Costa Rica with females guarding 1– 4 larvae on the upper surfaces of leaves and covering offspring with the body when disturbed. When females were removed, larvae were preyed on by ants or wandered away ( Choe 1989).
Proseicela Chevrolat sp. , New Record ( Figure 5C View Figure 5 , Table 1). Author RW observed subsociality on Sanchezia sp. (Acanthaceae) at two sites in Peru: Loreto Province, Palo Seco village, along Itaya River, near Iquitos, 150 m, understorey of altered mature forest, 29.V.2007, female guarding four larvae ( Figure 3C); and Picuroyacu, 150 m, understorey of altered mature forest, 17.IX.2009: female guarding six larvae.
Tribe Gonioctenini , Subtribe Gonioctenina
Gonioctena Gistel comprises 88 species found in the Nearctic, Palearctic, and Oriental regions. Known hosts are in Salicaceae , Betulaceae , Fagaceae , Rosaceae , and
Ulmaceae ( Mardulyn et al. 1997) View in CoL . The reproductive strategies of Gonioctena species combine oviparity, ovoviviparity, and viviparity with solitary and subsocial behaviours ( Waloff and Richards 1958; Takizawa 1976; Kudo and Hasegawa 2004). For example, Gonioctena springlovae (Bechyné) is ovoviviparous but solitary ( Kimoto and Takizawa 1994), Gonioctena sibirica (Weise) is ovoviviparous and subsocial ( Takizawa 1976; Kudo et al. 1995), and Gonioctena pallida (Linnaeus) is viviparous and solitary ( Selman 1988). Larvae exhibit gregariousness and chemical defences ( Takizawa 1976) and adults tend to be aposematically coloured.
Four Holarctic species exhibit maternal care ( Figure 6 View Figure 6 ): Gonioctena rufipes (Degeer) (as Phytodecta rufipes Dejean ; Lühmann 1940; Goidanich 1956; Hinton 1981), Gonioctena viminalis (L.), Gonioctena japonica Chujo and Kimoto (Kudo and Ishibashi 1995) and Gonioctena sibirica (Weise) in Japan (Kudo et al. 1995). Females of Gonioctena sibirica larviposit, then guard 20– 30 larvae that complete development in about 20 days. When disturbed, these gregarious larvae synchronously raise their abdomens and evert glands with defensive secretions. Pupation is solitary and underground. Females of G. rufipes deposit ~ 30 larvae ( Lühmann 1940) and guard them until instar I – II, then instar III larvae migrate away from the mother. The mother guards by sitting at the leaf petiole while her brood feeds; she feeds little herself. Mother and offspring migrate to new leaves together, and she always maintains a defensive posture – on the petiole, blocking pedestrian intruders. Apparently, the female cannot identify her own brood as she guards aggressively when switched to other broods. Females are courted while they are guarding and will copulate.
Tribe Phyllocharitini
Pterodunga Daccordi comprises a single species, Pterodunga mirabile Daccordi ( Figure 7 View Figure 7 , Table 1), documented on two species of Proteaceae in Garradunga, Queensland, Australia ( Reid et al. 2009; Hasenpusch, pers. commun.). Adults appear mainly during the dry season, from May to November, with peak populations in August–September. Females guard up to nine larvae and mothers actively herd larvae to maintain the aggregation, and produce defensive gland secretions when disturbed. These larvae lack defence glands; pupation is underground. The lack of a spermatheca, presence of mature embryos in the female, and lack of egg bursters typical of instar I larvae suggest that this species is ovoviviparous.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Mesomphaliini Hope 1840
| Chaboo, Caroline S., Frieiro-Costa, Fernando A., Gómez-Zurita, Jesús & Westerduijn, Rob 2014 |
Acromis
| Chaboo & Frieiro-Costa & Gómez-Zurita & Westerduijn 2014 |
Labidomera suturella
| Chevrolat, Reid 2009 |
Pterodunga
| Daccordi 2000 |
Gonioctena japonica
| Chujo and Kimoto (Kudo and Ishibashi 1995 |
Platyphora nigronotata (Stål) (
| Medeiros and Vasconcellos-Neto 1994 |
Platyphora microspina Bechyné
| Bechyne 1954 |
Paraselenis
| Spaeth 1913 |
Paraselenis
| Spaeth 1913 |
Omaspides (O.) abbreviata
| Baly 1872 |
Omaspides (Omaspides) abbreviata
| Baly 1872 |
Platyphora
| Gistel 1857 |
Platyphora
| Gistel 1857 |
Omaspides (O.) augusta
| Boheman 1856 |
Omaspides (Omaspides) pallidipennis
| Boheman 1854 |
Proseicela
| Chevrolat 1837 |
Cassidinae
| Gyllenhal 1813 |
Chrysomelinae
| Latreille 1802 |