Aetana Huber, 2005
|
publication ID |
https://doi.org/ 10.5852/ejt.2015.162 |
|
DOI |
https://doi.org/10.5281/zenodo.14452402 |
|
persistent identifier |
https://treatment.plazi.org/id/0390E827-603C-FF9D-95D1-F8A247F4F99C |
|
treatment provided by |
Jeremy |
|
scientific name |
Aetana Huber, 2005 |
| status |
|
Aetana Huber, 2005 View in CoL View at ENA
Aetana Huber, 2005a: 72–73 View in CoL .
Type species: A. omayan Huber, 2005 View in CoL .
Diagnosis
Even though the cladistic analysis identifies only two synapomorphies for Aetana , the genus is fairly easily distinguished from the putatively closest relatives by the following characters: retrolateral trichobothrium on leg 1 very proximal (at <5% of tibia length) and presence of curved hairs on tibiae and / or metatarsi (both in contrast to Spermophora , Khorata Huber, 2005 , Savarna , and an undescribed genus from Sarawak, below called ‘Gen.n. Borneo’); sternum not dark, retrolateral trichobothrium on male palpal tibia in very distal position, and presence of epiandrous spigots (all in contrast to Khorata , Savarna , and ‘Gen.n. Borneo’); male legs without spines and male palpal coxa unmodified (both in contrast to ‘Gen.n. Borneo’); ALS with only two spigots, epigynal plate without external pair of pockets, and female genitalia without unpaired posterior pocket (all in contrast to Spermophora ). Most characters previously thought to be diagnostic ( Huber 2005a) are rendered invalid due to the newly described species.
Description
Male
MEASUREMENTS. Total body length ~2.5–4.5 (smallest species in A. kinabalu group; largest species in A. omayan group); carapace width 0.9–1.8; leg 1 length ~27–44; tibia 1 length ~6.0–11.0; tibia 2/tibia 4 length 0.92–1.08; tibia 1 L/d ~55–95 (the largest species, A. omayan , has the relatively thickest legs; the smallest species, A. gaya , has the relatively thinnest legs).
COLOR. In life mostly ochre-gray with brown and black marks (e.g., Figs 51–56 View Figs 51–56 , 105–110 View Figs 105–110 ), only A. libjo Huber , sp. nov. and A. baganihan Huber , sp. nov. with light brown to orange prosoma and palps ( Figs 8–12 View Figs 6–12 ); sternum never dark; legs usually with indistinct darker rings on femora (subdistally) and tibiae (proximally and subdistally); darker rings missing in A. libjo Huber , sp. nov. and A. baganihan Huber , sp. nov.
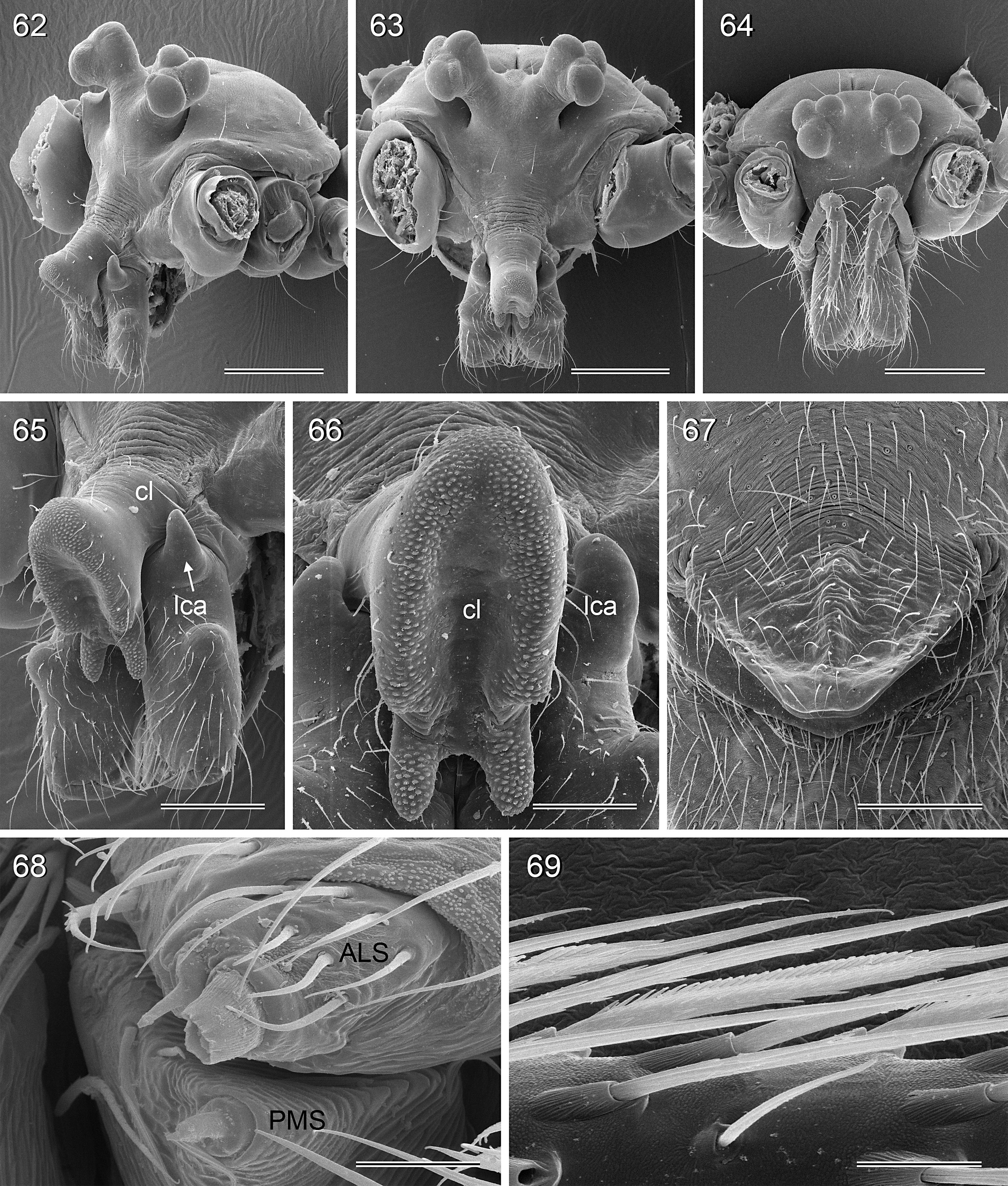
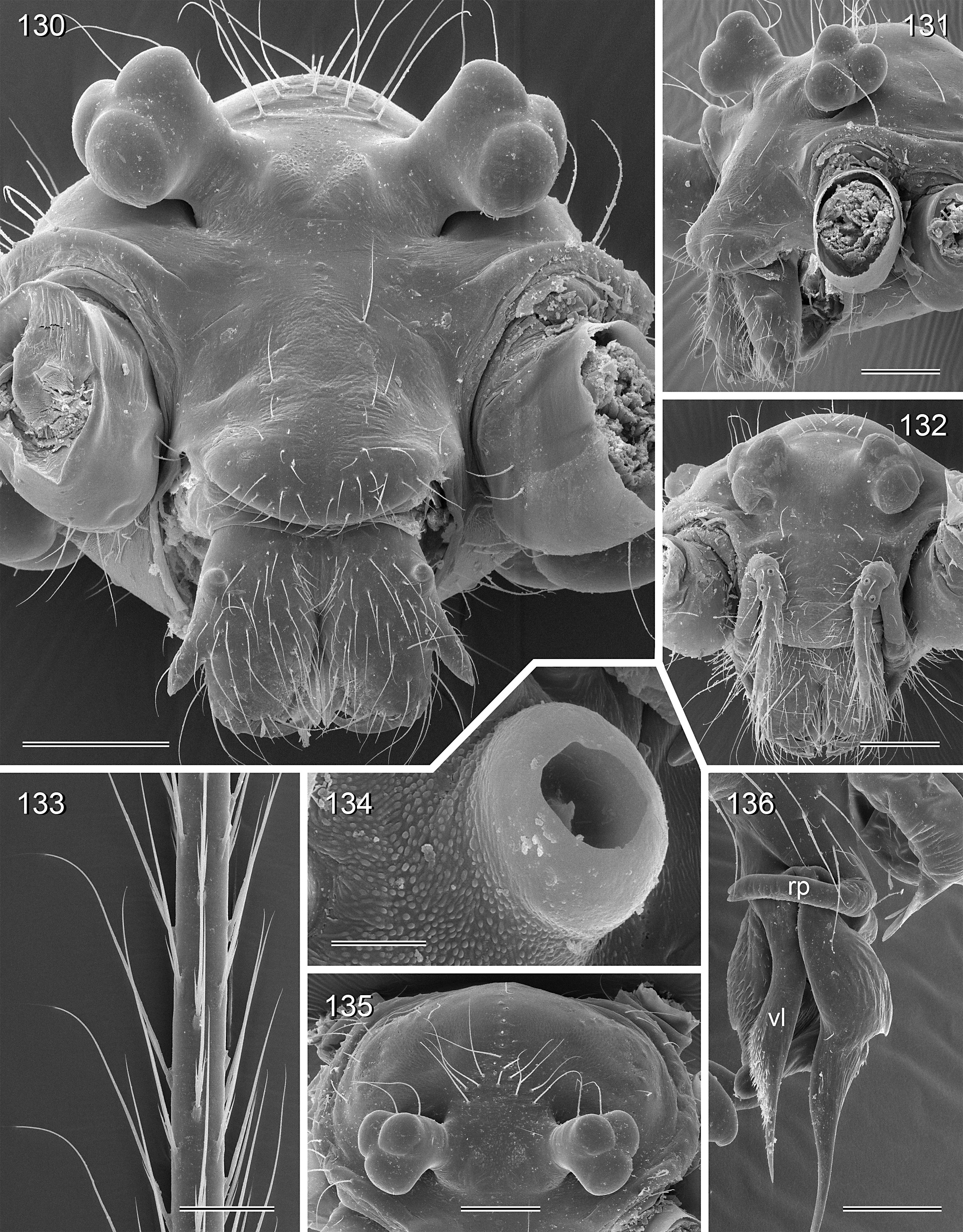
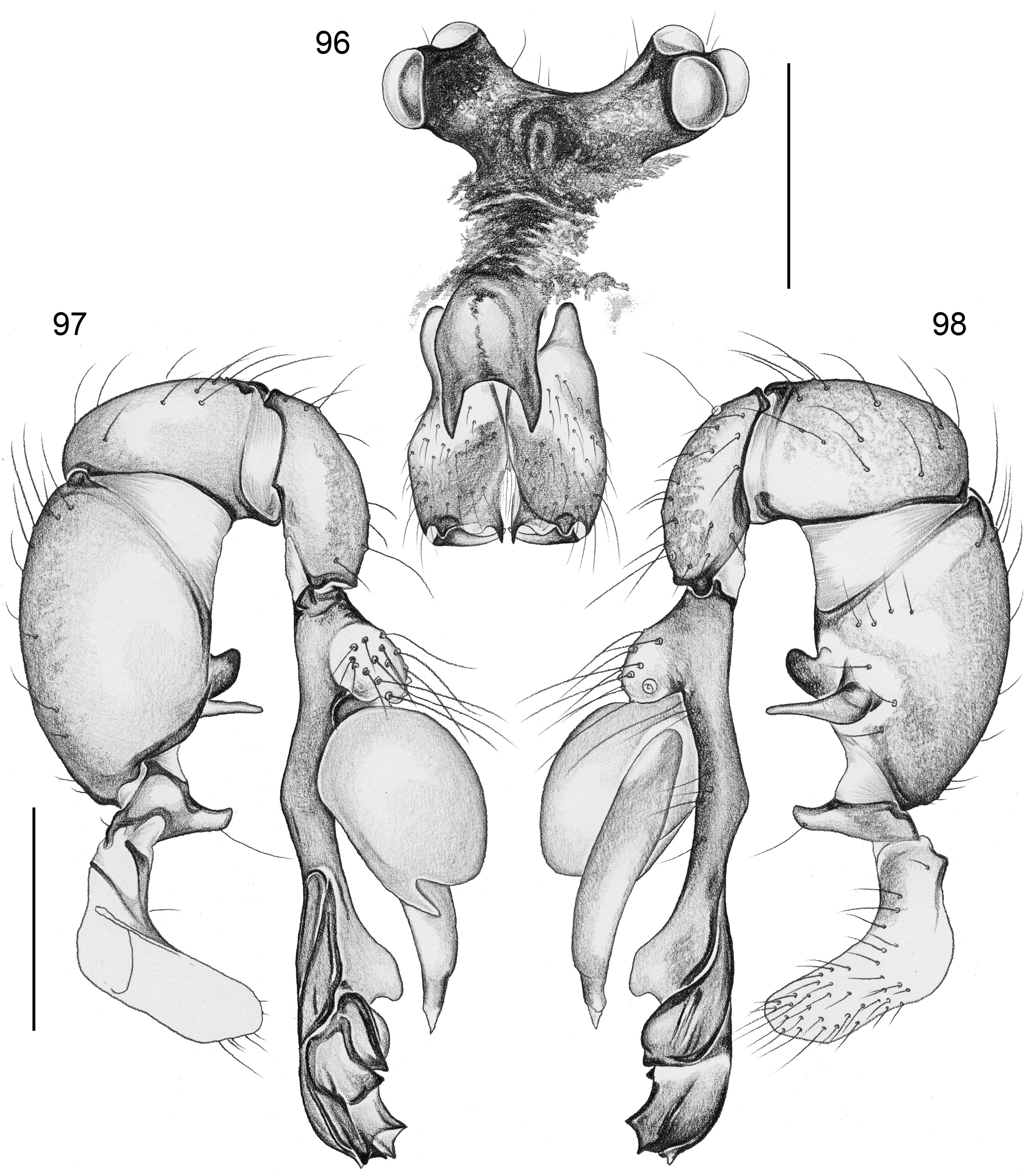
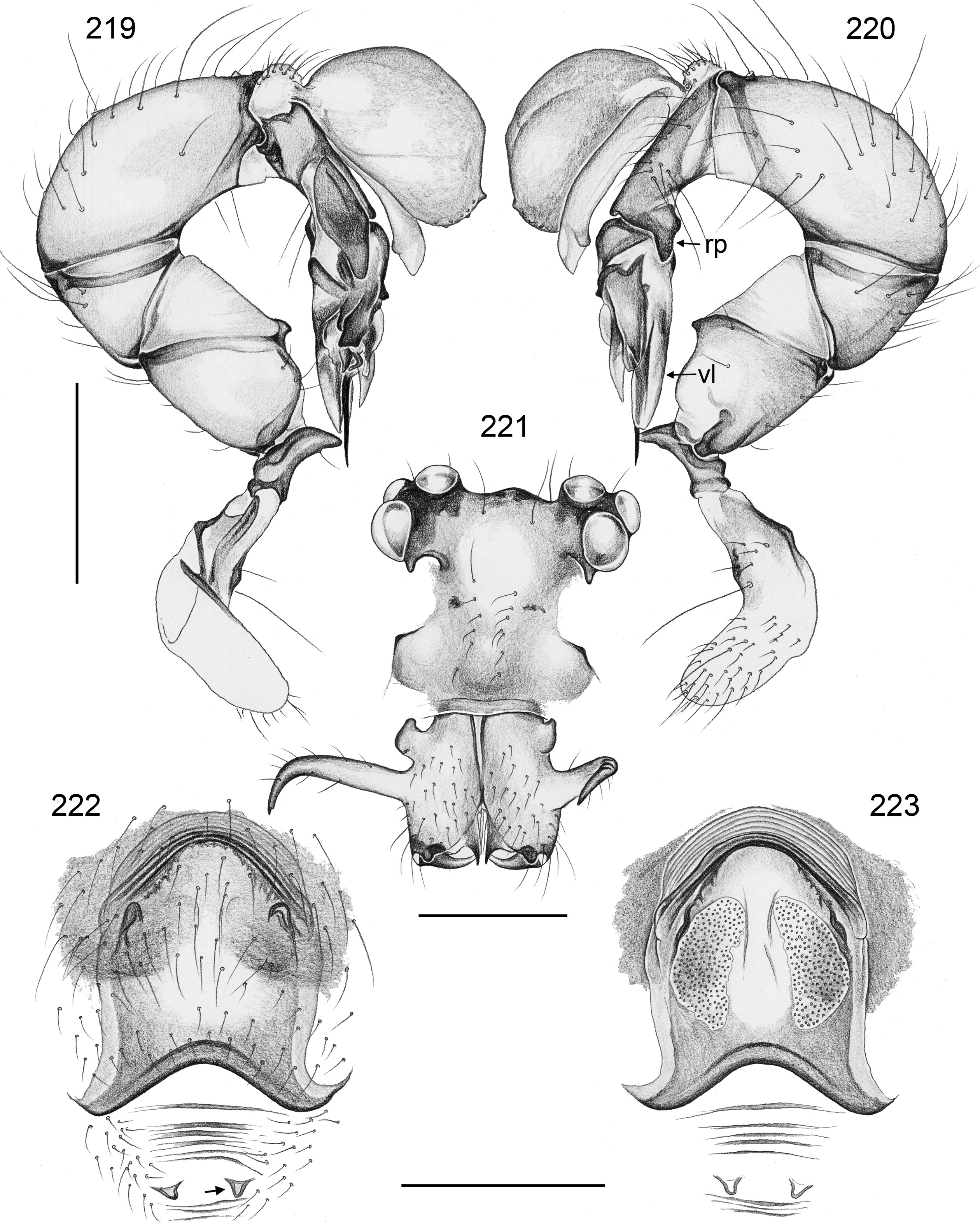
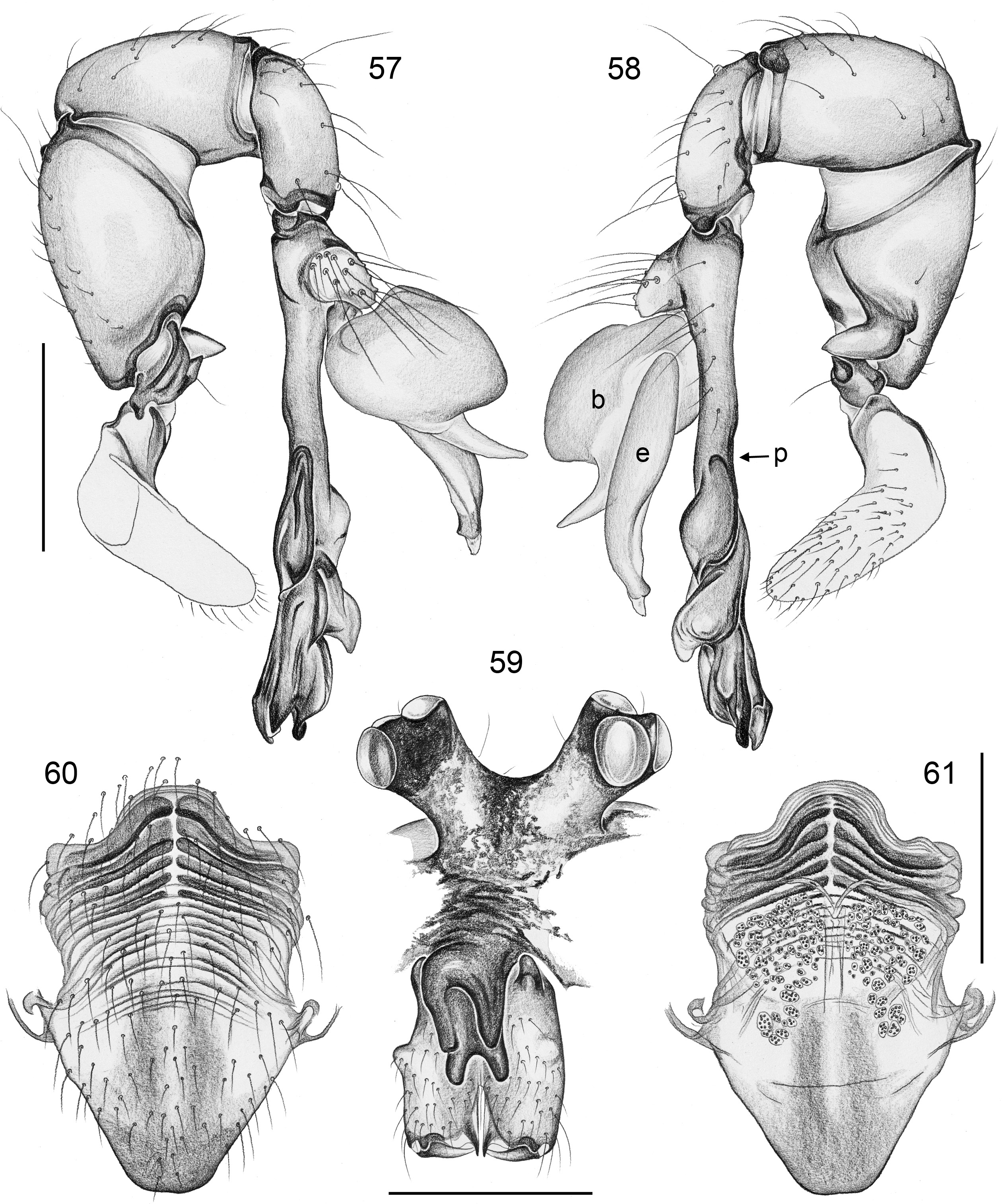
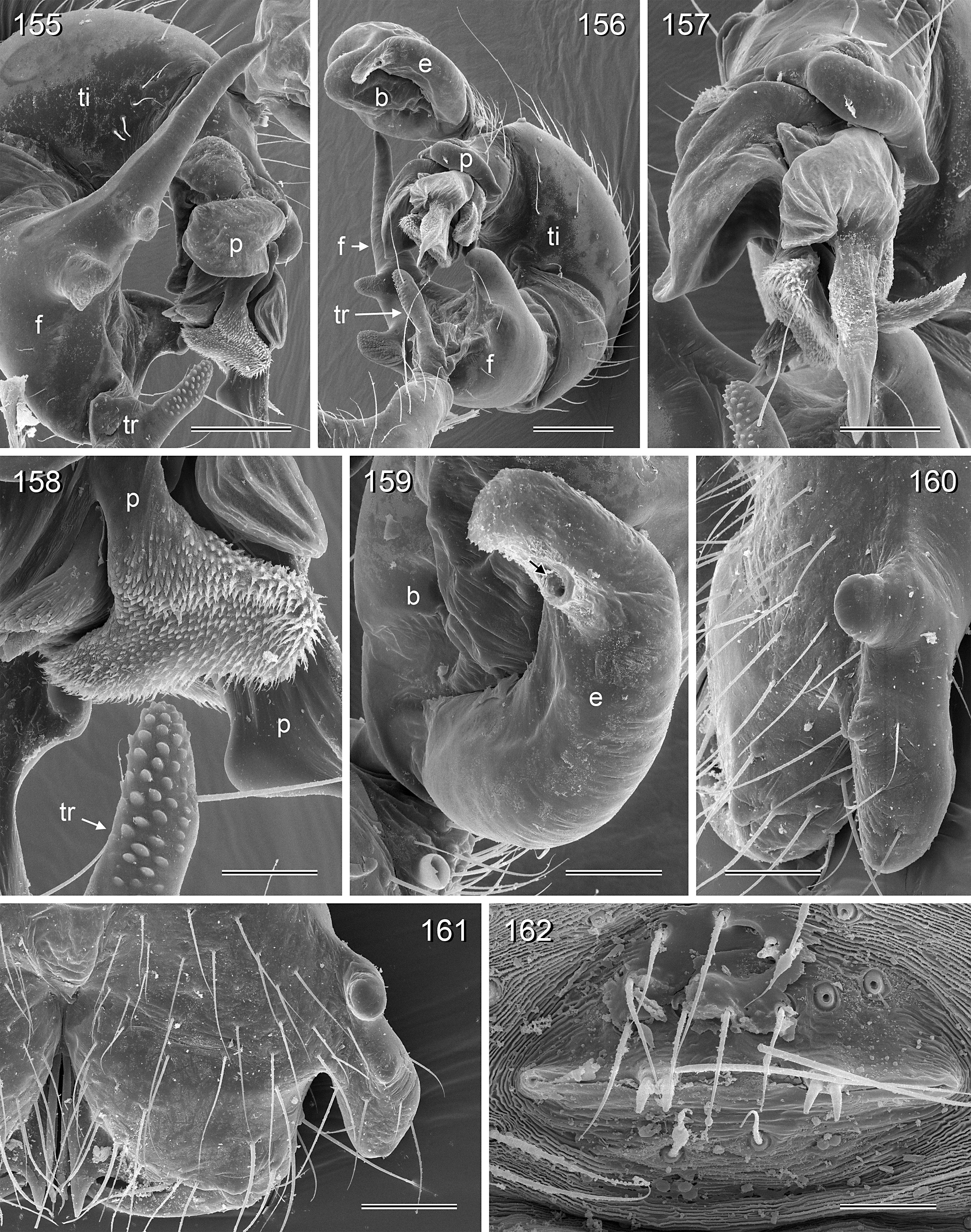
BODY. Carapace either with shallow median furrow restricted to frontal part ( Figs 63–64 View Figs 62–69 , 198 View Figs 195–203. — 195–197 ) or without furrow ( Figs 118 View Figs 118–125 , 130 View Figs 130–136 ); ocular area raised, eye triads on short stalks directed toward lateral, in some species with median process ( Figs 77 View Figs 75–79 , 96 View Figs 96–98 ) or with pair of processes arising from near ALE ( Figs 221 View Figs 219–223 , 226 View Figs 224–228 ). AME absent. Clypeus high, either unmodified, with small paired processes ( Fig. 191 View Figs 189–194. — 189–193 ), with large median process ( Figs 59 View Figs 57–61 , 77 View Figs 75–79 ), or with indistinct lateral ridges ( Figs 221 View Figs 219–223 , 226 View Figs 224–228 ). Abdomen from slightly longer than high ( Figs 51–56 View Figs 51–56 ) to almost cylindrical ( Figs 8 View Figs 6–12 , 102 View Figs 99–104 ), pointed at spinnerets. Male gonopore with four epiandrous spigots in all species examined with SEM (e.g., Figs 37 View Figs 30–37 , 143 View Figs 137–144 , 162 View Figs 155–162 ); each ALS with large widened spigot and pointed spigot, without further cylindrically shaped spigots ( Figs 68 View Figs 62–69 , 210 View Figs 204–212 ); PMS with two spigots each.
CHELICERAE. Very variable, distal apophyses ranging from short processes in frontal position ( Figs 15 View Figs 13–15 , 40 View Figs 38–44. — 38–40 ) to long processes in lateral position ( Figs 191 View Figs 189–194. — 189–193 , 226 View Figs 224–228 ), absent in A. kiukoki group; proximal apophyses usually present, large in A. kiukoki group ( Fig. 66 View Figs 62–69 ), absent in A. ocampoi group ( Figs 15 View Figs 13–15 , 40 View Figs 38–44. — 38–40 ); chelicerae without modified hairs; without stridulatory ridges.
PALPS. Coxa unmodified; trochanter usually with one retrolateral to ventral process, sometimes provided with scales or teeth ( Fig. 158 View Figs 155–162 ), in A. ocampoi View in CoL group fused to femur ( Figs 14 View Figs 13–15 , 27 View Figs 26–29 ); trochanter in some species with additional prolateral apophysis ( Fig. 189 View Figs 189–194. — 189–193 ); femur rarely simple ( Fig. 27 View Figs 26–29 ), usually with one or more processes, in A. kinabalu group with complex set of up to four processes (e.g., Figs 150–151 View Figs 150–154 ); patella either triangular in lateral view or ventral side longer than usual (e.g., Figs 14 View Figs 13–15 , 58 View Figs 57–61 ); tibia either of usual shape (e.g., Figs 14 View Figs 13–15 , 127 View Figs 126–129 ) or rather small and slender ( A. kiukoki group; e.g., Fig. 58 View Figs 57–61 ), with retrolateral trichobothrium in very distal position (close to tibia-tarsus joint; Figs 27 View Figs 26–29 , 114 View Figs 113–117 ); palpal tarsus small, usually with capsular tarsal organ ( Figs 134 View Figs 130–136 , 203 View Figs 195–203. — 195–197 ), exposed in A. libjo Huber , sp. nov. ( Fig. 35 View Figs 30–37 ) (not clear in A. ocampoi Huber , sp. nov. and A. baganihan Huber , sp. nov.); procursus usually complex with proximal and distal parts connected by membranous hinge; procursus in A. kiukoki group unusually long ( Figs 58 View Figs 57–61 , 76 View Figs 75–79 ), in A. ocampoi group reduced to simple semi-transparent process ( Figs 14 View Figs 13–15 , 27 View Figs 26–29 ); bulb either with weakly sclerotized embolus as only process ( Figs 114 View Figs 113–117 , 159 View Figs 155–162 ), in A. kiukoki group with additional short membranous process ( Figs 58 View Figs 57–61 , 75 View Figs 75–79 ), in A. ocampoi group with additional novel processes ( Figs 13–14 View Figs 13–15 , 26–27 View Figs 26–29 ).
LEGS. Without spines; with curved hairs on tibiae and/or metatarsi ( Fig. 133 View Figs 130–136 ); usually without or with few vertical hairs, only in A. libjo Huber , sp. nov. and A. baganihan Huber , sp. nov. with one dense row retrolatero-dorsally on each tibia; retrolateral trichobothrium on tibia 1 very proximal (at 2–4% of tibia length), prolateral trichobothrium absent on tibia 1, present on other tibiae. Tarsus 1 usually with ~25–30 pseudosegments, fairly distinct distally; tarsus 4 with single row of ventral comb-hairs of a modified Belisana - type (cf. Huber & Fleckenstein 2008; Figs 32 View Figs 30–37 , 69 View Figs 62–69 , 82 View Figs 80–86 , 209 View Figs 204–212 ).
Female
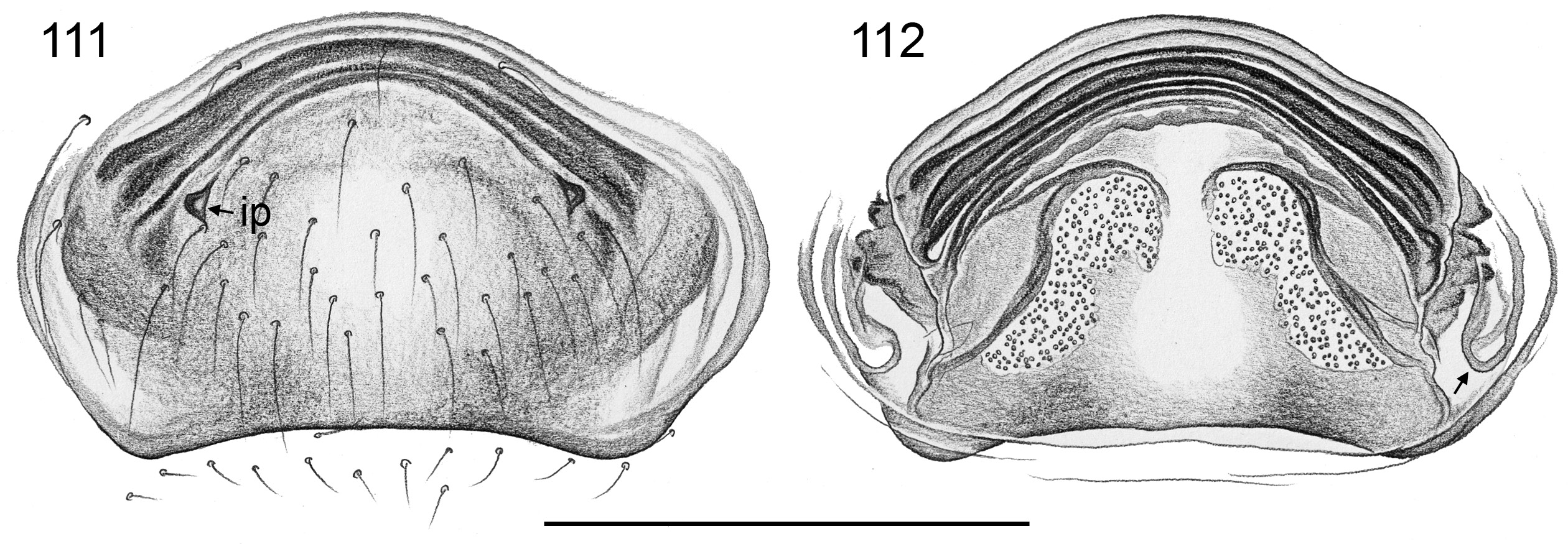
Similar to male but eye triads on lower humps ( Figs 64 View Figs 62–69 , 80 View Figs 80–86 , 119 View Figs 118–125 ) and closer together than in male (sexual dimorphism low in A. ocampoi View in CoL group; in other groups, eye triads in females often less than half as wide apart as in males); clypeus and chelicerae unmodified; legs slightly shorter than in males (tibia 1 ~4.0–9.0). Females in A. omayan group with stridulatory apparatus between prosoma (elongate median plate on carapace posteriorly; Figs 186–188 View Figs 178–188 ) against abdomen (indistinct hairless area frontally). Epigynum weakly to heavily sclerotized, sometimes with scape of variable length and shape ( Figs 28 View Figs 26–29 , 60 View Figs 57–61 ), never with external pair of pockets in anterior epigynal plate but in A. kinabalu and A. omayan groups sometimes with internal sclerotized pockets originating from ventral wall of uterus externus ( Figs 111 View Figs 111–112 , 192–194 View Figs 189–194. — 189–193 ), and in A. omayan group with pair of membranous external pockets in posterior epigynal area ( Figs 192, 194 View Figs 189–194. — 189–193 , 222 View Figs 219–223 ). Internal genitalia with pair of pore plates, in A. libjo Huber , sp. nov. and A. baganihan Huber , sp. nov. with unique median membranous structure ( Figs 29 View Figs 26–29 , 44 View Figs 38–44. — 38–40 ); in A. kiukoki group with distinctive serrated ridges ( Figs 61 View Figs 57–61 , 73 View Figs 70–74 , 79 View Figs 75–79 ).
Monophyly and relationships
Morphologically, the monophyly of Aetana appears weakly supported. Only two characters support this node ( Fig. 1 View Fig ), both of them without homoplasy within the taxa included in the matrix but with considerable homoplasy among more distant Pholcinae relatives: (1) the very proximal position of the retrolateral trichobothrium on the leg tibiae (char. 30); and (2) the presence of curved hairs on leg tibiae and/or metatarsi (char. 31). However, preliminary analyses of molecular data (including many more potential close relatives) (A. Valdez-Mondragón, D. Dimitrov, B.A. Huber, unpubl. data) consistently support the monophyly of Aetana with high support values.
The sister group of Aetana appears much better supported by morphology. Four characters suggest that three genera together ( Khorata , Savarna , and ‘Gen.n. Borneo’) are sister to Aetana . This, however, is in conflict with our preliminary molecular data that suggest a closer relationship between Aetana and East Asian Spermophora (incl. S. estebani ) than between Aetana and Khorata , Savarna , and ‘Gen.n. Borneo’. Such a close relationship with East Asian Spermophora was actually proposed in the original description ( Huber 2005a), at that time without a formal cladistic analysis.
Within Aetana , four species groups receive strong support both from the morphological analysis herein ( Fig. 1 View Fig ) and from our preliminary molecular data. Conflict exists regarding relationships among these groups. The present analysis supports a sister group relationship between the Aetana kinabalu and A. omayan groups, and at least one of the three characters supporting this node is a unique feature (the long retrolateral membranous process on the procursus; char. 21). Our preliminary molecular data do not strongly support any sister group relationships among the four species groups within Aetana .
Natural history
Most species were collected in forests, from well protected spaces close to the ground, under rocks and logs, in small holes and cavities. Few species occur higher in the vegetation, even in places directly reached by the sun, most notably the closely related A. libjo Huber , sp. nov. and A. baganihan Huber , sp. nov. and the putatively close relatives A. kinabalu and A. lambir Huber , sp. nov. An exception is the type species A. omayan , which was mainly collected in a cave but also among rocks in a treeless ravine.
All species seem to build simple domed sheet webs (like most other pholcids studied), but the sheets are unusually strongly domed in A. libjo Huber View in CoL , sp. nov. and A. baganihan Huber , sp. nov., and in A. kinabalu and A. lambir Huber , sp. nov. a second sheet occurs a few cm under the main sheet.
In six cases, two species were found to share a locality, sometimes in different microhabitats (e.g., at Baganihan: A. kiukoki Huber , sp. nov. near the ground; A. baganihan Huber , sp. nov. among vegetation), sometimes in what seemed to be identical microhabitats (e.g., at Mt. Banahaw: A. manansalai Huber , sp. nov. and A. banahaw Huber , sp. nov., both close to the ground).
In most species, males and females were often found together sharing a web. This was never observed in A. libjo Huber , sp. nov. and A. baganihan Huber , sp. nov., where males and females were sometimes observed very close to each other but in separate webs.
When disturbed, Aetana spiders tend to run toward the periphery of the web rather than to vibrate in the ‘typical’ pholcid way. Some do then vibrate vigorously for a very short time before becoming motionless and pressing their body against the substrate; others stop vibrating and start to gently move the abdomen in circles ( A. libjo Huber , sp. nov. and A. baganihan Huber , sp. nov.). The closely related A. poring Huber , sp. nov. and A. indah Huber , sp. nov. barely reacted to disturbance.
Composition
The genus now includes 18 described species. The high species turnover in the Philippines and in northern Borneo, together with the wide distribution of one species group ( A. omayan group; Philippines to Fiji Islands) and the huge sampling gaps in eastern Indonesia, New Guinea, and east to Fiji suggest that at least several dozen further species are likely to exist.
Distribution
Ranging from northern Borneo and the Philippines to Fiji ( Fig. 2 View Fig ). Most gaps and missing records are probably due to lack of adequate sampling, but the absence of records from two areas might reflect real absence. First, our intensive collecting in western Sarawak (at seven localities ranging from the Pueh foothills in the west to Niah in the east) did not result in a single specimen of Aetana , while many specimens were collected at all eight localities in eastern Sarawak (east of Niah) and Sabah. Second, northern Australia is relatively well sampled, but a revision of all the material available in collections (Huber 2001) did not reveal any Aetana .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Aetana Huber, 2005
| Huber, Bernhard A., Nuñeza, Olga M. & Ung, Charles Leh Moi 2015 |
Aetana
| Huber B. A. 2005: 73 |