Zyzzyzus rubusidaeus, Brinckmann-Voss, Anita & Calder, Dale R., 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3666.3.9 |
|
publication LSID |
lsid:zoobank.org:pub:767E3CD7-615D-4C62-9F84-BBA7D39676C9 |
|
DOI |
https://doi.org/10.5281/zenodo.6152128 |
|
persistent identifier |
https://treatment.plazi.org/id/038EC03A-2819-2041-FF0E-39E90E5FCF66 |
|
treatment provided by |
Plazi |
|
scientific name |
Zyzzyzus rubusidaeus |
| status |
sp. nov. |
Zyzzyzus rubusidaeus View in CoL , sp. nov.
Tubulariidae .—Millen, 1985: 15.
Corymorpha sp.—Harbo, 1999: 32; 2011: 46.—Austin, 2000: 58, 60, 62, 63.
Material examined. Holotype: Kuldekduma Island, Weynton Passage, Broughton Strait, British Columbia, Canada, 50°35.299’N, 126°50.046’W, depth 60 feet (18 m), 30 March 2012, on rock amongst barnacles and sponges, mature polyp, 2.5 cm high (preserved in 4% formaldehyde; specimen part of a larger aggregation covering about 8 cm 2), with female (?) gonophores, coll. Neil McDaniel, ROMIZ B3957.
Paratypes: Kuldekduma Island, Weynton Passage, Broughton Strait, British Columbia, Canada, 50°35.299’N, 126°50.046’W, depth 60 feet (18 m), 30 March 2012, on rock amongst barnacles and sponges, several mature and juvenile polyps (part of the same aggregation that included the holotype), adults with female gonophores, coll. Neil McDaniel, ROMIZ B3958.—Kuldekduma Island, Weynton Passage, Broughton Strait, British Columbia, Canada, 50°35.299’N, 126°50.046’W, depth 60 feet (18 m), 30 March 2012, on rock amongst barnacles and sponges, mature and immature hydranths (part of the same aggregation that included the holotype), coll. Neil McDaniel, RBCM 2012-00234-001.
Additional material: Pearse Reefs, Weynton Passage, Broughton Strait, British Columbia, Canada, depth 60 feet (18 m), 23 July 2012, several mature and juvenile polyps, adults with female gonophores, coll. Steve Lacasse, ROMIZ B3959.—Plumper Island, Weynton Passage, depth 60 feet (18 m), 9 October 2012, several polyps, all male, coll. Steve Lacasse, RBCM 012-00277-001.
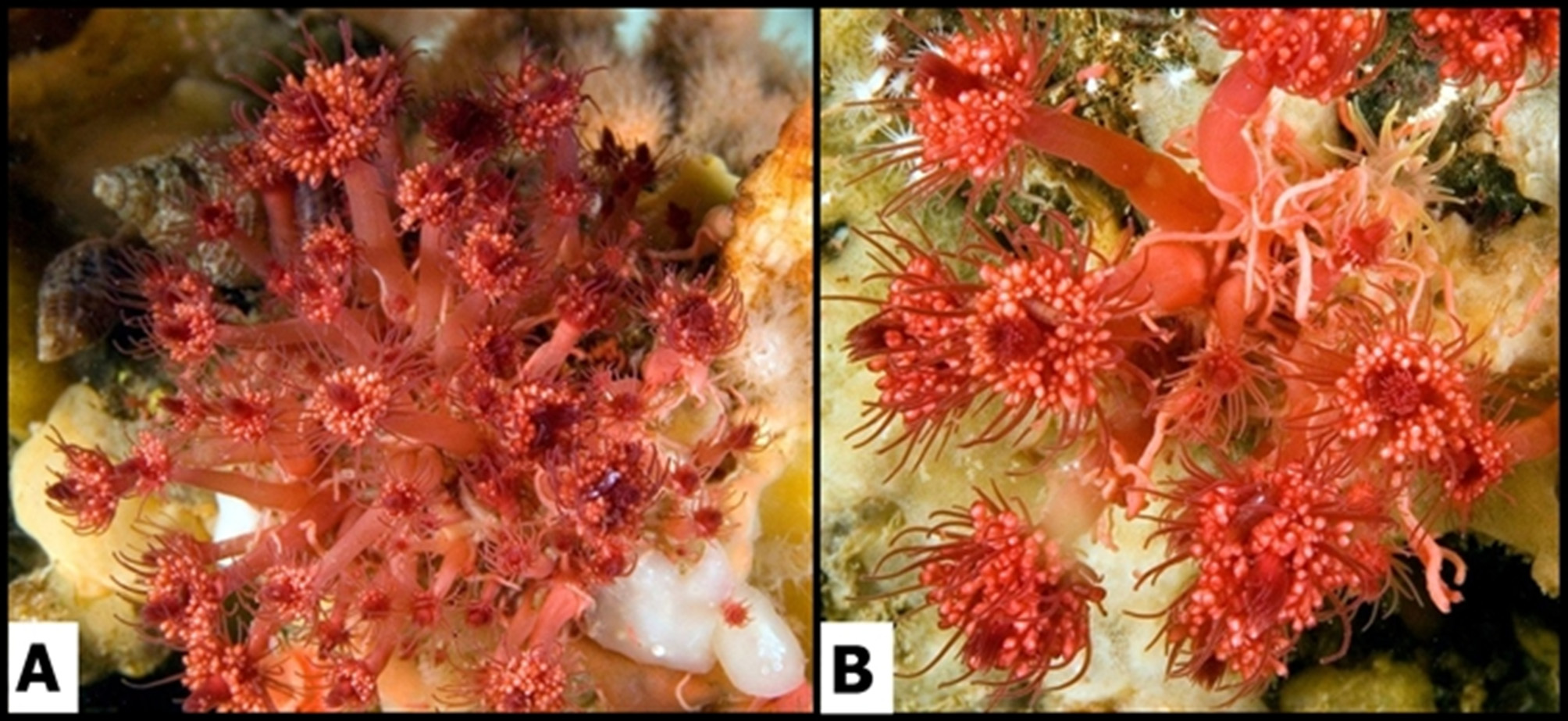
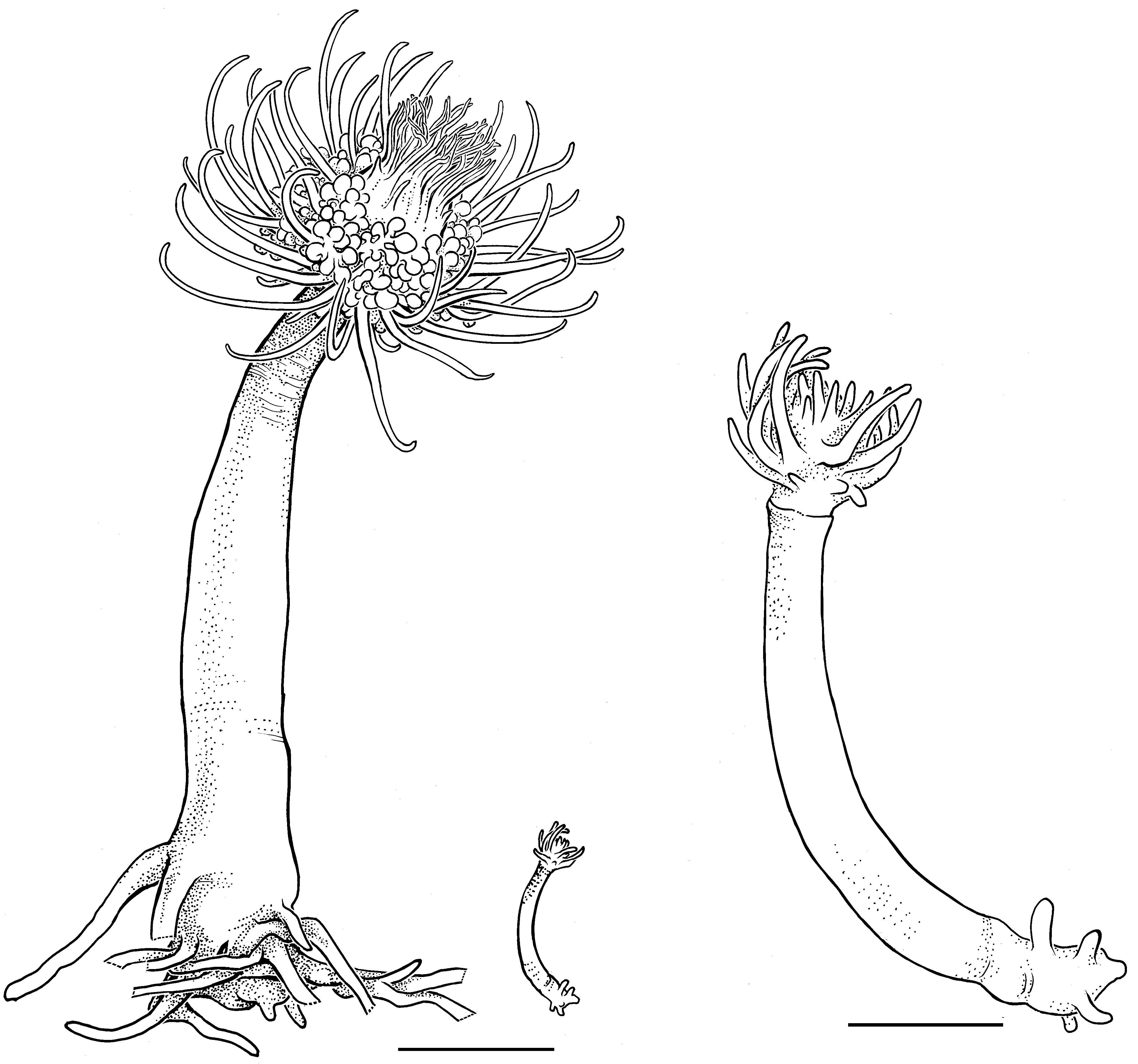
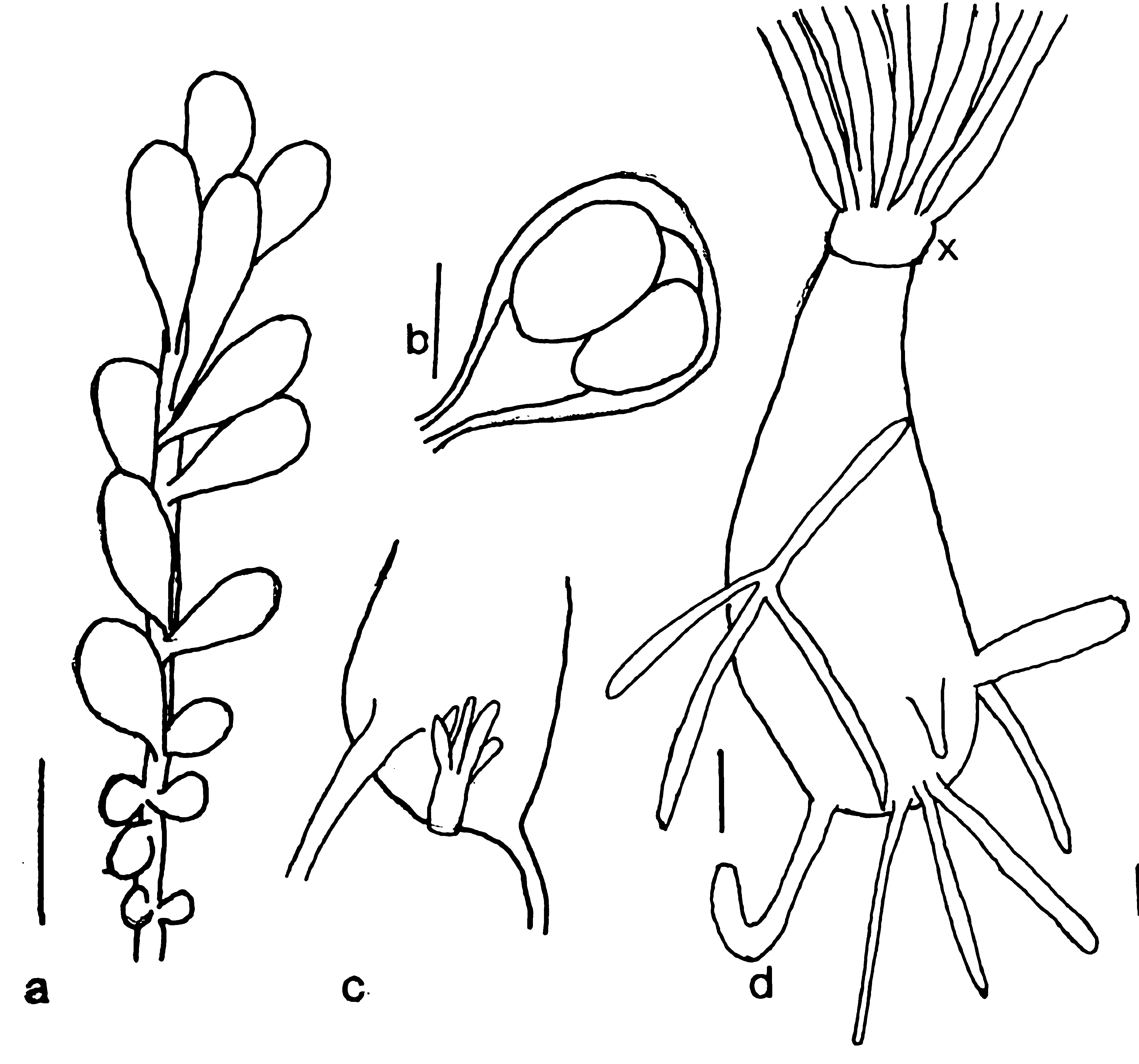
Description ( Figs. 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 ). Hydroids occurring in colony-like aggregations of polyps composed of adults and numerous juveniles in all stages of development; adults up to 5 cm high and 5 mm wide (hydranth body without tentacles; preserved specimens). Aggregations varying from those having one adult polyp plus one juvenile to others comprising numerous adult and juvenile polyps and covering several cm2 ( Figs.1 View FIGURE 1 A, B). Polyps within an aggregation loosely bound together by tubers growing from lower parts of hydrocauli ( Fig. 2 View FIGURE 2 ); tubers either thin and forming a loose, web-like mass resembling a hydrorhiza, or consisting of two or three thick, finger-like projections from base of hydrocaulus clasping around substrate and thereby anchoring polyps; mass formed by entwined tubers not very stable, such that groups of one to several hydranths easily separated; anastomosing of tubers not observed. New polyps developing either from lower part of hydrocaulus, or from thicker tubers often adjacent to an existing hydrocaulus ( Fig. 3 View FIGURE 3 c), or sprouting from thinner tubers on hydrorhiza; less frequently, new polyps arising from outer side of perisarc of hydrocaulus, the origin of such polyps presently being unclear. Perisarc thin, transparent, often inconspicuous or lacking in upper section of hydrocaulus; empty perisarc tubes present in some parts of colony. Hydrocaulus wide, often rounded and broad at base, narrowing distally and widening slightly below aboral tentacle whorl. Dissected hydrocauli filled with more or less dense parenchymatic tissue, walls coursed by small but numerous peripheral endodermal canals ( Fig. 4 View FIGURE 4 ). Hydranths vasiform, with oral and aboral whorls of tentacles, short neck area visible below aboral tentacles ( Fig. 3 View FIGURE 3 d); interior of hydranth with a large oral chamber above cushion of vacuolated cells. Aboral tentacles filiform, 26–40 in number in adult polyps, arranged in a narrow band made up of two or three concentric rings. Oral tentacles filiform, 50–60, arranged in five irregular whorls, shortest ones surrounding mouth. Blastostyles about 20 per hydranth, of varying lengths, usually not longer than hydranth, arising from hydranth between oral and aboral tentacle whorls. Gonophores oval, without radial canals or tentacles, arising from small pedicels on all sides of blastostyles ( Fig. 3 View FIGURE 3 a). Female gonophores ( Fig. 3 View FIGURE 3 b) with a few large cell masses thought to be embryos, but no actinulae found in any of a large number of gonophores examined from two different collections.
Cnidome ( Fig. 5 View FIGURE 5 ). Stenoteles, small (on oral and aboral tentacles, hydranth body, and gonophores; n = 10): 8.2–10.2 x 6.3–8.3 μm (undischarged).—Stenoteles, large (on oral and aboral tentacles, hydranth body, and gonophores; n = 10): 10.9–11.8 x 9.0–10.1 μm (undischarged).—Desmonemes (on oral and aboral tentacles, hydranth body, and gonophores; n = 10): 7.5–8.7 x 6.0–7.1 μm (undischarged).—Microbasic euryteles (on oral and aboral tentacles, hydranth body, and gonophores; n = 10): 11.9–14.8 x 8.1–10.0 μm (undischarged).—Basitrichs (on oral and aboral tentacles, hydranth body, and gonophores; n = 9): 7.0–11.3 x 2.0–3.4 μm (undischarged).— Isorhizas, large (on hydranth body and gonophores; n = 10): 15.0–19.5 x 6.9–9.0 μm (undischarged).—Isorhizas, slender (on gonophores; n = 2): 8.1–9.8 x 1.6–1.8 μm (undischarged).
Remarks. Within the genus Zyzzyzus Stechow, 1921 (Petersen 1990; Campos et al. 2007), now comprising seven species, Z. rubusidaeus most closely resembles Z. robustus Petersen 1990 . Unlike in the latter species, (1) perisarc surrounding the zooid is thin and transparent rather than thick, stiff, and conspicuous, (2) aboral tentacles are scattered within a narrow band at the base of the hydranth rather than being arranged in a single whorl, (3) gonophores arise from blastostyles on simple pedicels that sometimes split only at their end instead of occurring on seven or eight very short, stout branches (Petersen, 1990: 184). Hydroids of Z. rubusidaeus are a striking raspberry colour which fades but little in formalin, whereas Z. robustus is described as a “rich yellow brown” (Petersen 1990: 184). Unlike in Z. rubusidaeus , which may grow in dense, colony-like aggregations, hydroids of Z. robustus and others of the genus are described as “solitary” and appear to grow in a more scattered pattern (Campos et al. 2007, Figs. 1 View FIGURE 1 a, b).
We do not know how polyp aggregations in Z. rubusidaeus are formed, or whether each aggregation represents a clone. Juvenile polyps in our material most commonly arose either from the tubers or from proximal regions of the hydrocauli of fully-developed polyps; less often they arose from the outer layer of perisarc enveloping the hydrocaulus of larger specimens. It remains unclear, however, whether such young polyps arose by asexual budding, by settlement of sexually-generated larval stages from the same aggregation, by settlement of such larvae from adjacent aggregations, or by a combination of these methods. It is therefore questionable whether they constitute a colony, usually defined in Hydrozoa as an assemblage of interconnected polypoid and/or medusoid individuals derived asexually from a single larval stage (Cornelius 1995; Bouillon et al. 2006; Nawrocki & Cartwright 2012). It is also uncertain whether they constitute “aggregated colonies” in the sense of Stepanjants et al. (2002), defined as an association of polyps that are loosely connected basally yet have common coenosarc.
As noted by Cornelius (1995), zoological terminology does not yet precisely cover the variety of phenomena grouped within the term “colony.” Indeed, Nawrocki & Cartwright (2012) reported a different kind of colony formation in Ectopleura larynx (Ellis & Solander, 1786) , a hydroid belonging to the same family ( Tubulariidae ) as Zyzzyzus rubusidaeus . Colonies in that species were formed through fusion of polyps that had been generated sexually, rather than through asexual means. However aggregations of polyps are established in Z. rubusidaeus , and whether they can be defined as colonies or not, observed polyp clusters in the species likely afford those adaptive advantages attributed to a colony over solitary animals on hard substrates, especially increased effectiveness in competition for space (Jackson 1977; Osman 1977; Nawrocki & Cartwright 2012).
Zyzzyzus rubusidaeus was first mentioned in the literature as “the raspberry hydroid” in a published abstract by Millen (1985). She noted that it was preyed upon by both an aeolid nudibranch and a stenothoid amphipod. In describing that previously unknown nudibranch, as Cuthona punicea, Millen (1986) noted that its prey was an “…undescribed, wine-red hydroid…” The species has been illustrated in Pacific coast guidebooks (e.g. Harbo 1999, 2011, as Corymorpha sp.), and it was listed, again as Corymorpha sp., in a work on rare and endangered invertebrate species of British Columbia (Austin 2000).
Although actinula larvae are reported in the life cycle of other species of the genus Zyzzyzus , we have been unable to confirm their existence in Z. rubusidaeus . Oval, whitish masses believed to be embryos were visible in female gonophores of our material, but their nature is still obscure ( Fig. 3 View FIGURE 3 b).
Distribution. Although guidebooks claim the distribution of the “raspberry hydroid” (as Corymorpha sp.) to be from Alaska to central California, we could confirm its occurrence only for Weynton Passage in Broughton Strait, and Discovery Passage off Quadra Island (Rick Harbo, 1999, 2011 and personal information), all in British Columbia.
Etymology. The Latin specific name rubusidaeus refers to the raspberry colour and appearance of the species, and to the common name “raspberry hydroid” frequently given to this conspicuous species (Millen 1985; Harbo 1999, 2011; Austin 2000) by biologists and divers in the vicinity of the type locality (Broughton Strait, off Vancouver Island, Canada, NE Pacific).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Hydroidolina |
|
Order |
|
|
Family |
|
|
Genus |