Mystus cyrusi, Esmaeili & Sayyadzadeh & Zarei & Eagderi & Mousavi-Sabet, 2022
|
publication ID |
https://doi.org/10.11646/zootaxa.5099.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:FB28F74A-DD16-48D7-9A1A-F5675886DFE4 |
|
DOI |
https://doi.org/10.5281/zenodo.6310504 |
|
persistent identifier |
https://treatment.plazi.org/id/2F6C449C-C2A2-4F8F-95E8-B21DDB4C2CAB |
|
taxon LSID |
lsid:zoobank.org:act:2F6C449C-C2A2-4F8F-95E8-B21DDB4C2CAB |
|
treatment provided by |
Plazi |
|
scientific name |
Mystus cyrusi |
| status |
sp. nov. |
Mystus cyrusi , new species
( Figs. 2–7 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 )
Holotype. Female, ZM-CBSU J2901, 94.2 mm SL, Iran: Fars prov.: Darab city, Forg region , Qalatooyeh (Ghalatooye) village , Qalatooyeh Spring , Kol River drainage, 28°10’16.8”N, 55°14’42.5”E. GoogleMaps
Paratypes. ZM-CBSU J2902, 4, 65–92 mm SL, same data as holotype. —ZM-CBSU J2906, 3, 79–98 mm SL, Fars prov.: Darab city, Qalatooyeh region, Kol River drainage, 28°10’14.75”N, 55°14’59.90”E GoogleMaps .— ZM-CBSU J2909, 5, 68–98 mm SL; Iran: Fars prov.: Darab city, Forg region, Qalatooyeh village , Qalatooyeh Spring , Kol River drainage, 28°10’16.8”N, 55°14’42.5”E GoogleMaps . — ZM-CBSU J2914, 2, 71–95 mm SL, Fars prov.: Darab city, Golabi spring, Kol River drainage, 28°47’14.72”N, 54°22’14.93”E GoogleMaps .
Diagnosis. Mystus cyrusi belongs to a group of species having a long adipose-fin base and short maxillary barbel and lacking a caudal spot. Mystus cyrusi is distinguished from M. pelusius , the only other species of the genus in the Tigris-Euphrates River system in the Middle East, by a combination of characters: maxillary barbel short, not reaching to beyond of pelvic fin (vs. extends as far as anal fin in some females in M. pelusius ), smaller adipose-fin base (30.8–37.4% SL), and with a steeper sloping at its origin (vs. longer, 37.6–45.6% SL and with a more gently sloping in M. pelusius ), greater head depth (16.64–21.9% SL vs. 12.6–16.59% SL in M. pelusius ), greater caudalpeduncle depth (10.3–12.5% SL vs. 8.7–10.5 in M. pelusius ) and fewer total gill rakers (12–14 with a mode of 12 vs. 14–17 in M. pelusius ).
Description. See figures 2–4 for general appearance and Table 4 View TABLE 4 for morphometric data. Body short or moderately elongated, rounded anteriorly and compressed posteriorly, dorsal profile slightly convex, rising steeply from tip of snout to dorsal-fin origin, ventral profile almost straight.
Head short and flattened. Snout obtuse. Mouth subterminal and transverse. Eyes anteriorly situated, not visible from below ventral surface of head, moderately large with free circular margins. Four pairs of barbels, one each of maxillary and nasal and two of mandibular. Nasal barbel extend back to eye, maxillary barbel short, not reaching to beyond of pelvic fins (extending to vertical through third or fourth dorsal-fin ray, in some specimens reaching to origin of pelvic fin). Inner mandibular barbel not reaching to pectoral-fin base, outer one reaching. Gill openings wide, extending from post temporal to beyond isthmus. Skin smooth. Lateral line complete, midlateral in position. Total gill rakers 12–14, (mode of 12). Dorsal fin with II–III, 7 rays and a convex margin, usually anterior branch of fin rays longer than other branches; dorsal-fin spine short, straight, and slender, posterior edge without serrations. Pectoral fin with I, 7–8 rays, pectoral-fin spine stout and stronger than the dorsal spine, serrated with 8–10 antrorse teeth on the inner margin, the number increasing with size. Pectoral-fin margin straight anteriorly and convex posteriorly. Pelvic-fin origin slightly posterior to vertical through posterior end of dorsal-fin base, with I, 5 rays and slightly convex margin; tip of appressed fin not reaching to anal-fin origin. Anal fin with rays difficult to separate into branched and unbranched (perhaps I–IV, 8–10 rays). Adipose-fin origin at end of dorsal fin when appressed and ended in origin of caudal fin with free end. Adipose-fin origin starts with a steeply sloping and terminate at its highest portion. Caudal fin deeply forked; upper lobe larger than lower lobe.
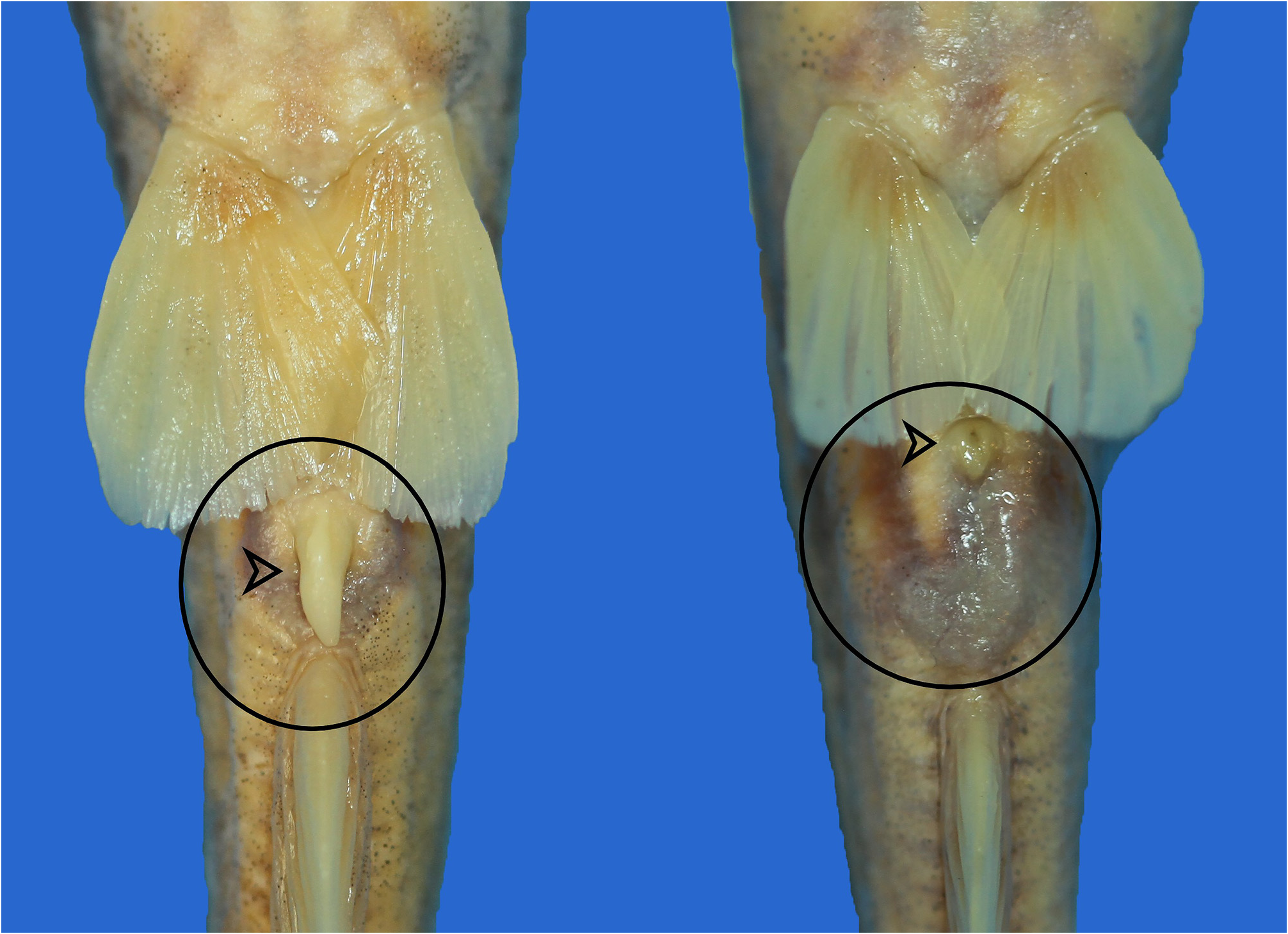
Sexual dimorphism. Males with slightly long genital papilla. Females with rounded genital opening ( Fig. 5 View FIGURE 5 ).
Coloration. Dorsal surface of head and body pale brown to olivaceous. Dark humeral spot and black spot at base of dorsal fin present. Ventral surfaces of head and body dirty white. Dorsal and anal fins with melanophores on rays and membranes and so these fins darker than other fins. Margin of adipose fin narrowly black. Caudal fin with black margin. Three (sometimes two), narrow, white stripes on flank, one along and one each above and below lateral line. Stripe below dorsal and adipose fins narrower than others. Barbels whitish, somewhat darker dorsally ( Fig. 6 View FIGURE 6 ).
Distribution and Habitat. Mystus cyrusi is currently known from three localities ( Fig. 7 View FIGURE 7 ), Qalatooyeh ( Fig. 8 View FIGURE 8 ) and Golabi Springs ( Fig. 9 View FIGURE 9 ), and Shur River, Kol River drainage which flow to the Straits of Hormuz, Persian Gulf, in southern Iran. Golabi Spring has a high water temperature all year, 22.8°C was measured in August and 22.4°C in December. The water is typically clear with some green coloration. The spring pool is circular with a depth of less than 1.5 m. The bottom is pebbles, gravel or mud. Phragmitis sp. (Poaceae) and Juncus sp. (Juncaceae) are the dominant riparian vegetation. The spring run is short and has a moderately swift flow, emptying into cemented channels being used for agricultural purposes.
Conservation. Mystus cyrusi occurs in low numbers. Hence, care should be taken to conserve the populations. Drought and introductions of alien fishes, particularly Gambusia holbrooki and Neotropical convict cichlid, Amatitlania nigrofasciata (see Esmaeili et al. 2013), are major threats to this endemic fish species.
Etymology. The species is named for Cyrus the Great, king of Persia.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |