Bathyraja abyssicola ( Gilbert, 1896 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5142.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:AB36996C-74D9-416A-94C2-106345FAFF75 |
|
DOI |
https://doi.org/10.5281/zenodo.6958247 |
|
persistent identifier |
https://treatment.plazi.org/id/038987A4-933E-FFEF-73D5-FACCCDA4092F |
|
treatment provided by |
Plazi |
|
scientific name |
Bathyraja abyssicola ( Gilbert, 1896 ) |
| status |
|
Bathyraja abyssicola ( Gilbert, 1896) View in CoL
Figures 1–2 View FIGURE 1 View FIGURE 2 ; Table 1 View TABLE 1 , 8–9 View TABLE 8 View TABLE 9
Deepsea Skate
Raja abyssicola Gilbert, C.H., 1896: 396 , Pl. 20 [United States Commission of Fish and Fisheries, Report of the Commissioner] v. 19 (for 1893) (art. 6). Holotype: USNM 48623 About USNM (disintegrated). Off Queen Charlotte Island GoogleMaps , British Columbia, Canada, 52°39'30"N, 132°38'00"W, Albatross station 3342, depth 1,588 fathoms.
Raja abyssicola: Goode & Bean, 1895: 509 (listed); Jordan & Evermann, 1896: 76 (compiled); Garman, 1913: 344 (compiled); Fowler, 1930: 502 (listed); Jordan et al., 1930: 26 (listed); Grey, 1956: 100 (compiled); Clemens & Wilby, 1961: 90, fig. 28 (description, range); Grinols, 1965: 25 (listed); Miller & Lea, 1972: 46 (description, figure, key); Quast & Hall, 1972: 4 (listed); Hart, 1973: 55 (description, figure, range, reference).
Bathyraja abyssicola: Stehmann, 1978: 53 View in CoL (reference); Amaoka et al., 1983: 54–55 (description, figure; range; remarks); Masuda et al., 1984: 13; Stehmann, 1986: 263; Zorzi & Anderson, 1988: 93; McAllister, 1990: 34 (listed); Castro-Aguirre & Espinosa Pérez, 1996: 27 (listed); McEachran & Dunn, 1998: 286 (listed); Castro-Aguirre et al., 1999: 63 (listed); Dolganov, 1999: 429; Compagno, 1999 (listed): 488; Sheiko & Fedorov, 2000: 15; Hoff, 2002: 145 (description, figure, range); Mecklenburg et al., 2002: 102 (listed); Nakabo, 2002: 168 (key, listed); Ebert, 2003: 193 (description, distribution); Fedorov et al., 2003: 15; Stehmann, 2005a: S35, S53; Stevenson & Orr, 2005: 73–80 (description, figure); Ebert & Compagno, 2007: 116 View Cited Treatment (listed); Ebert & Davis, 2007: 3–4 View Cited Treatment (egg case description); Parin et al., 2014: 29 (listed); Dyldin, 2015: 61 (listed); Weigmann, 2016: 90 (listed); Last et al., 2016: 373 (listed, figure); Kells et al., 2016 (figure): 78; Ebert et al., 2017: 21, 58, 68 (description, distribution, key, listed); Cerutti-Pereyra et al., 2018: 87 (description, figure, range); Dyldin & Orlov, 2018: 168 (listed); Ehemann et al., 2018: 24 (listed); Burton & Lea, 2019: 32 (listed); Calle-Morán et al., 2020: 246 (listed); Dyldin & Orlov, 2021: 58 (listed).
Diagnosis. Large, rhomboidal skates (to at least 1,570 mm TL) with a triangularly shaped disc (width 44.2–63.7% TL), long head length (19.7–27.8% TL), and rounded pectoral apices; claspers very long and slender, tip of clasper conspicuously bulbous, large and wide pseudosiphon present, length 20.0% of clasper, possesses a distinct, curved pseudorhipidion, inner surface has a defined V-shaped cleft; ventral lobe with a rounded projection; teeth in 27–39 and 24–34 rows on upper and lower jaw, respectively; pectoral radials 82; pelvic fins 19; total vertebrae 139; dorsal and ventral surface of disc with prickly dermal denticles; thorns present on dorsal surface of disc, males with a welldeveloped alar thorns, malar thorns absent, middorsal thorns weak or absent (0–2), scapulars usually absent, nuchal thorns strong (2–4), tail thorns moderate (15–30), down the length of tail, interdorsals weak or obsolete (0–1); dorsal coloration dark brown or black-grey, occasionally with small dark blotches scattered on body, pectoral fin edges darker than the rest of body, pelvic fins often with whitish anterior tips; ventral coloration darker than dorsal surface, often with white coloration around mouth, gills, and cloaca.
Description. A large, flabby-bodied skate with a rhomboidal disc, 1.0–1.6 times as broad as long; disc length very triangular; disc length and width large; anterior margin strongly triangular, moderately concave in adult males, straight to moderately convex beside and just forward of eyes; apex rounded; posterior margin convex and broadly rounded; free rear tip broadly rounded. Head length 27.5–27.8% TL and preorbital snout length very long 12.4–16.8% TL. Preoral length relatively long 12.0–17.3% TL and prenarial length substantially large 9.8–14.3% TL. Snout tip narrowly pointed, possessing no fleshy process at apex. Eye length relatively small 2.0–4.6% TL. Spiracles average 1.9–3.4% TL, oval shaped. Nasal curtain length large 2.7–9.1% TL, width average 6.3–9.7% TL, its posterior margin fringed at the corners; anterior margin of curtain lobe-like. Internarial distance 5.5–8.6% TL; gills roughly equivalent in length, except for a smaller fifth gill slit; first gill slit length 1.6–2.6% TL; fifth gill slit length 1.3–2.4% TL; distance between first gill slits 12.0–18.0% TL, and distance between fifth gill slits 10.0–13.7% TL. Upper jaw moderately well arched, possessing a symphysis; lower jaw convex. Teeth similar in both jaws; teeth unicuspid, with a strong, bluntly pointed posteriorly directed cusp; arranged in longitudinal rows; upper and lower teeth moderate in number (27–39 and 24–34, respectively).
Pelvic fins large, posterior lobe 7.2–12.8% TL, anterior lobe 4.9–10.4% TL, and inner margin deeply incised 2.9–8.6% TL. Tail moderately long 52.1–61.7% TL, rather slender; wider at base, tapering to the first dorsal fin origin, not expanded in the middle. Lateral tail fold small, 16.5–22.4% TL, similar in both sexes; not obviously broader at any point along its length. Dorsal fins moderate in size and shape, first dorsal fin slightly taller than second dorsal fin, 1.8–3.1% TL and 1.9–2.6% TL, respectively; bases of both dorsal fins are similar in length, 3.1–4.0% TL for first dorsal fin and 2.7–4.2% TL for second dorsal fin; anterior margins of both fins concave, apices rounded; free rear tip rounded; interdorsal space short 0.4–1.7% TL, with larger individuals having a larger interdorsal space, rear tip of first dorsal fin not overlapping base of second dorsal fin. Caudal fin small, low, height 0.2–1.0% TL; its dorsal margin weakly concave; not connected to second dorsal fin by a small membranous ridge. Tail relatively long 52.1–61.7% TL.
Middorsal, nuchal, interdorsal, and tail thorns present, males with a well-developed set of alar thorns; malar thorns absent; thorns vary in size, from very short to well-developed. Middorsal thorns weakly developed or absent (0–2), causing a discontinuous row from scapular region to first dorsal fin; scapulars usually absent; nuchal thorns strongly developed (2–4); tail thorns moderate (15–30) and go down the length of the tail; interdorsal thorns weakly developed and small in number (0–1). Alar thorn patches possess 3–6 rows and 20–23 columns on both pectoral fins. No multiple rows of thorns on body.
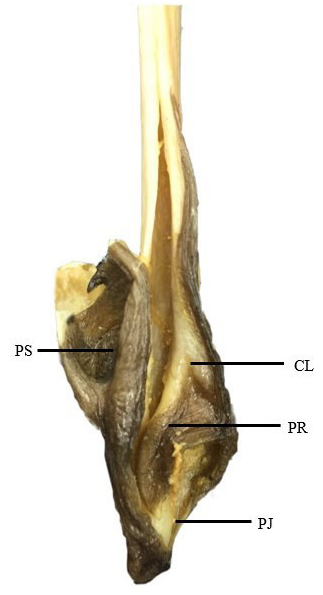
Mature claspers very slender and long, base length 0.9–1.9% TL, inner length 20.4–30.7% TL, tip of clasper conspicuously bulbous and wider than the rest of the structure ( Figure 3 View FIGURE 3 ). Clasper fully developed with squared tip, its length 35.7–49.7% of tail length. Very large and wide pseudosiphon present near the outer lateral edge of dorsal lobe, its length 20.0% of clasper length; inner surface of dorsal lobe with a distinct, curved pseudorhipidion; inner surface possesses a clearly defined V-shaped cleft; ventral lobe possesses a rounded projection.
Clasper skeleton consists of 3 dorsal terminal, 1 accessory terminal, ventral terminal and axial cartilages; dorsal terminal 1 squared with a notch on the inner edge; dorsal terminal 1 curves around the axial onto ventral side and connected with ventral terminal, forming the relatively large pseudosiphon externally; tip of dorsal pointed, forming the pseudorhipidion externally; ventral terminal long, leaf-shaped, and overlying the tip of ventral marginal and accessory terminal 1; tip of ventral marginal pointed, forming a rounded projection externally.
Dermal denticles possess 3–4 points to the base and are well-developed on posterior third of the dorsal surface; denticles on the first dorsal fin long, needle-like, posterior-oriented; denticles on head stouter and strongly curved ( Figure 4 View FIGURE 4 ). Possesses very fine prickles on the dorsal surface. Dermal denticles found on both dorsal and ventral surfaces, including the tail.
Length of rostral cartilage 51.1% of cranial length; prefontanelle rostral length 46.7%; cranial width 61.4%; least interorbital width 18.5%; length of anterior prefontanelle 14.1%; length of rostral appendices 16.2%. Rostral cartilage abruptly tapering near its broad base; rostral appendices long, its length 38.4% of the length of rostral cartilage; anterior fontanelle spade-shaped ( Figure 5 View FIGURE 5 ).
Coloration. Dorsal coloration dark brown or black to grey, occasionally with small dark blotches scattered on body. Snout semi-translucent. Spiracles pale white to pink. Pectoral fin edges are darker than the rest of the body; pelvic fins darker, often with whitish anterior tips. Ventral coloration slightly darker than dorsal surface; often possessing white coloration around mouth, gills, and cloaca; irregular pale blotches and numerous dark spots often scattered on body. Claspers brown to grey; cloaca sometimes possesses a dark ring around it. Thorns on dorsal surface are very pale. Coloration after preservation is a uniform brown both dorsally and ventrally.
Egg case description. The egg cases are large (108–111 mm TL), light golden brown in color, with a coarse surface, due to the presence of rasp-like denticles. Cases possess a distinct groove between the lateral keel and the case, a feature that is absent in all other ENP skate egg cases. Horns are present at the corners, with the anterior horns being more robust than posteriors, both sets becoming flat and thread-like at tips ( Ebert & Davis 2007).
Distribution. Bathyraja abyssicola has been confirmed as occurring in the North Pacific, specifically from the Bering Sea, south to the Galapagos Islands ( Cerutti-Pereyra et al., 2018), and as far west as Japan ( Ebert, 2003). It occurs at depths of 362–2,906 m ( Ebert, 2003); the specimens included in this study were found no shallower than 968 m and not deeper than 1,212 m. The relatively shallow depth for the specimens in this study is due to the maximum survey depth, not because the species does not occur at those depths.
Biological notes. Size at maturity for males 109–120 cm TL; 145 cm TL for females. Size at birth is uncertain, although the smallest free-swimming specimen measured 19 cm TL ( Stevenson & Orr, 2005). Maximum is size is 135 cm TL and 157 cm TL for males and females, respectively ( Matta et al., 2006). It is a predator that feeds on annelids, cephalopods, crabs, shrimps, and bony fishes, with smaller individuals consuming more invertebrates ( Ebert, 2003).
Habitat. Inhabits deep waters, on the upper continental slope ( Ebert, 2003), often over fine sediment. Has been reported to have a preference for colder temperatures than its congeners ( Kuhnz et al., 2019)
Etymology. The species was named after the Greek abyssos, meaning bottomless, and cola, meaning living at depths, referring to its deep-sea habitat.
Comparisons. Bathyraja abyssicola is one of the largest bathyrajid species that occurs in the ENP and can easily be differentiated from the smaller-bodied species in its geographic range, including B. kincaidii and B. microtrachys . Additionally, the dorsal and ventral colorations of B. interrupta , B. kincaidii , and B. spinosissima easily distinguish those species from B. abyssicola . Head, preorbital, and prenarial lengths are significantly longer than all of its congeners (F 6,104 = 10.23, p <0.0001, F 6,104 = 12.41, p <0.0001, and F 6,104 = 16.24, p <0.0001, respectively). Furthermore, middorsal thorn count is significantly lower than its congeners (F 6,104 = 16.2, p <0.0001).
Bathyraja microtrachys is a smaller-bodied species that differs from B. abyssicola in its thorns counts and coloration. Bathyraja abyssicola possesses thorns in the middorsal and nuchal regions, whereas these thorns are absent in B. microtrachys . Bathyraja trachura shares a similar range and coloration to B. abyssicola , but lacks middorsal thorns, and has a significantly smaller interdorsal space and head length.
Bathyraja aleutica is also similar to B. abyssicola in size, coloration, and distribution. Bathyraja abyssicola possesses dark coloration on both sides, whereas B. aleutica possesses a predominantly pale ventral side. Furthermore, B. aleutica possesses a longer interdorsal space, shorter head length, and shorter preorbital snout. Bathyraja abyssicola has the longest snout to body size of any species in this study.
Remarks. Individuals seen in remotely operated vehicle footage have been found to face up-slope when at rest ( Kuhnz et al., 2019). Unlike some of its congeners, this species has a preference for swimming above the seafloor ( Kuhnz et al., 2019).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Bathyraja abyssicola ( Gilbert, 1896 )
| Knuckey, James D. S. & Ebert, David A. 2022 |
Bathyraja abyssicola:
| Dyldin, Y. V. & Orlov, A. M. 2021: 58 |
| Calle-Moran, M. D. & Bearez, P. 2020: 246 |
| Burton, E. J. & Lea, R. N. 2019: 32 |
| Cerutti-Pereyra, F. & Yanez, A. B. & Ebert, D. A. & Arnes-Urgelles, C. & Salinas-de-Leon, P. 2018: 87 |
| Dyldin, Y. V. & Orlov, A. M. 2018: 168 |
| Ehemann, N. R. & Gonzalez-Gonzalez, L. D. V & Chollet-Villalpando, J. G. & De La Cruz-Aguero, J. 2018: 24 |
| Ebert, D. A. & Bigman, J. S. & Lawson, J. M. 2017: 21 |
| Weigmann, S. 2016: 90 |
| Dyldin, Y. V. 2015: 61 |
| Parin, N. V. & Evseenko, S. A. & Vasil'eva, E. D. 2014: 29 |
| Ebert, D. A. & Compagno, L. J. V. 2007: 116 |
| Ebert, D. A. & Davis, C. D. 2007: 3 |
| Stevenson, D. E. & Orr, J. W. 2005: 73 |
| Ebert, D. A. 2003: 193 |
| Fedorov, V. V. & Chereshnev, I. A. & Nazarkin, M. V. & Shestakov A. V. & Volobuev, V. V. 2003: 15 |
| Hoff, G. R. 2002: 145 |
| Mecklenburg, C. W. & Mecklenburg, T. A. & Thorsteinson, L. K. 2002: 102 |
| Nakabo, T. 2002: 168 |
| Sheiko, B. A. & Fedorov, V. V. 2000: 15 |
| Castro-Aguirre, J. L. & Espinosa Perez, H. & Schmitter-Soto, J. J. 1999: 63 |
| Dolganov, V. N. 1999: 429 |
| McEachran, J. D. & Dunn, K. A. 1998: 286 |
| Castro-Aguirre, J. L. & Espinosa Perez, H. 1996: 27 |
| McAllister, D. E. 1990: 34 |
| Zorzi, G. D. & Anderson, M. E. 1988: 93 |
| Stehmann, M. F. W. 1986: 263 |
| Masuda, H. & Amaoka, K. & Araga, C. & Uyeno, T. & Yoshino, T. 1984: 13 |
| Amaoka, K. & Nakaya, K. & Araya, H. & Yasui, T. 1983: 54 |
| Stehmann, M. F. W. 1978: 53 |
Raja abyssicola:
| Hart, J. L. 1973: 55 |
| Miller, D. J. & Lea, R. N. 1972: 46 |
| Quast, L. C. & Hall, E. L. 1972: 4 |
| Grinols, R. B. 1965: 25 |
| Clemens, W. A. & Wilby, G. V. 1961: 90 |
| Grey, M. 1956: 100 |
| Fowler, H. W. 1930: 502 |
| Jordan, D. S. & Evermann, B. W. & Clark, H. W. 1930: 26 |
| Garman, S. 1913: 344 |
| Jordan, D. S. & Evermann, B. W. 1896: 76 |
| Goode, G. B. & Bean, T. H. 1895: 509 |