Rhamphostomella costata Lorenz, 1886
|
publication ID |
https://doi.org/10.11646/zootaxa.5131.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:CF550031-D6A9-48A3-A953-A1BD40C72F5E |
|
DOI |
https://doi.org/10.5281/zenodo.7628934 |
|
persistent identifier |
https://treatment.plazi.org/id/03892374-0B3A-332F-FF73-A9BE1D13FDAA |
|
treatment provided by |
Plazi |
|
scientific name |
Rhamphostomella costata Lorenz, 1886 |
| status |
|
Rhamphostomella costata Lorenz, 1886 View in CoL
( Figs 3 View FIGURE 3 , 31G–I View FIGURE 31 )
Cellepora scabra: Smitt 1868a, p. 30 (part), pl. 28, figs 186, 187.
Rhamphostomella costata Lorenz, 1886, p. 12 View in CoL , pl. 7, fig. 11 (mentioned as fig. 12 in the text).
Rhamphostomella costata: Hincks 1889, p. 426 View in CoL , pl. 21, figs 7, 8; Nordgaard 1905, p. 171, pl. 5, figs 21, 22; Kluge 1962, p. 537, fig. 375; 1975, p. 654, fig. 375; Osburn 1912a, p. 244, pl. 26, figs 62a, b, pl. 31, fig. 100; 1952, p. 426, pl. 50, fig. 7; Gostilovskaya 1978, p. 226, fig. 142; Androsova 1958, p. 171, fig. 102; Mawatari 1965, p. 619, text-figs 126c, d; Tarasova 1983, p. 25, fig. 31; Dick & Ross 1988, p. 67, pl. 5a; Ryland & Hayward 1991, p. 39, fig. 59.
Rhamphostomella scabra var. costata: Nordgaard 1929, p. 7 View in CoL .
Rhamphostomella magnirostris Canu & Bassler, 1928, p. 120 View in CoL , pl. 19, figs 5–7, text-figs 22e, f.
Additional references. Rhamphostomella costata: Nordgaard 1906, p. 30 View in CoL , 41; Kluge 1908a, p. 533; 1908b, p. 553; 1928, p. 257; 1929, p. 21; 1952, p. 160; 1953, p. 178; 1961, p. 141; 1964, p. 190; Osburn 1936, p. 542; 1955, p. 38; O’Donoghue & O’Donoghue 1923, p. 44; 1925, p. 105; 1926, p. 72; Gostilovskaya 1957, p. 455; 1968, p. 70; Kluge et al. 1959, p. 213; Hansen 1962, p. 41; Powell 1968a, p. 2310; 1968b, p. 257; Gontar 1980, p. 13; 1990, p. 132; 1992, p. 195; 1994a, p. 144, 145; 1994b, p. 106; 2010, p. 153; Mawatari & Mawatari 1981, p. 55; Dick & Ross 1986, p. 89; Denisenko 1988, p. 13; 1990, p. 39; 2008, p. 187; 2011, p. 14; 2013, p. 184; Gontar & Denisenko 1989, p. 354; Grishankov 1995, p. 48; Grischenko 1997, p. 174; 2002, p. 115; 2015, p. 40; Gontar et al. 2001, p. 195; Kuklinski 2002b, p. 203; 2009, p. 228; Kuklinski & Bader 2007b, p. 840; Denisenko & Kuklinski 2008, p. 48; Kuklinski & Taylor 2009, p. 497; Ostrovsky 2009, p. 206, fig. 78c; 2013, p. 8, fig. 2.41c; Foster 2010, p. 57.
Material examined. Lectotype: NHMW 92531 View Materials (=1884.II.50), three fragments of one colony initially encrusting shell of Chlamys islandica, L. Lorenz Collection , II Austro-Hungarian Polar Expedition, 1882–1883, Jan Mayen, depth 200–270 m, ollector F. Fischer . Paralectotype 1: NHMW 72990 View Materials (=1884.II.50), a few small fragments of one colony initially encrusting shell of Chlamys islandica, L. Lorenz Collection , II Austro-Hungarian Polar Expedition, 1882–1883, Jan Mayen, depth 200–270 m, collector F. Fischer. Paralectotype 2: NHMW 92532 View Materials (=1884.II.92), colony encrusting gastropod shell, L. Lorenz Collection, II Austro-Hungarian Polar Expedition, 1882–1883, Jan Mayen, depth 200–270 m, collector F. Fischer.
NHMUK 1911.10.1.1574A, colony encrusting bivalve shell, A.M. Norman Collection, HMS Valorous , 1875, Greenland, depth 104 m. NHMUK 1899.5 About NHMUK .1.273, three colony fragments, locality not given, T. Hincks Collection . NHMUK 2010.2 About NHMUK .9.6, one colony fragment, PIBOC Collection , RV Akademik Oparin , 14th Expedition , Stn 17, 12 August 1991, near Kodiak Island , Gulf of Alaska, Pacific Ocean, 57°58.2ʹ N, 151°07.5ʹ W, depth 83 m, Sigsbee trawl, collector A. V. Smirnov. GoogleMaps P. Kuklinski Collection, two colony fragments, RV Ivan Kireev , Russian-German Expedition Transdrift 1, Stn 48, 18 August 1993, Laptev Sea, 74°30.0ʹ N, 137°05.0ʹ E, depth 22 m, rock dredge, collectors M.K. Schmid and D. Piepenburg. GoogleMaps SMNH-1730, one colony fragment, Swedish Arctic Expedition , August 1861, Waygat Islands , Hinlopen Strait , Svalbard and Jan Mayen, 79°10.0ʹ N, 19°00.0ʹ E, depth 55 m, rocks. GoogleMaps SMNH-126202, one colony, Egedesminde ( Aasiaat), Disko Bay, Western Greenland, 68°42.0ʹ N, 52°45.0ʹ W, collector O. Torell. GoogleMaps SMNH-126208, four fragments, Swedish Arctic Expedition , 1861, Treurenberg Bay, west Spitsbergen, Svalbard and Jan Mayen, depth 67 m . SMNH-126585, one colony, Swedish Arctic Expedition , 1861, Waygat Islands ( Vaigattøyane), Hinlopen Strait, Svalbard and Jan Mayen, 79°10.0ʹ N, 19°00.0ʹ E, depth 55 m, stones. GoogleMaps SMNH-132267, four fragments of a single colony, Spetsberg Expedition, June 1864, Safehaven, Isfjorden, Spitsbergen, depth 55 m GoogleMaps . SMNH-178050, two colony fragments, Swedish Arctic Expedition , August 1861, Waygat Islands, Hinlopen Strait, Svalbard and Jan Mayen, 79°10.0ʹ N, 19°00.0ʹ E, depth 50 m, rocks GoogleMaps . USNM 11115 About USNM , nine colony fragments, Arctic Research Laboratory Collection ,? August 1948, Point Barrow , Alaska, Beaufort Sea, depth 99.9 m, collector G.E. MacGinitie. USNM 11148 About USNM , six colony fragments, Arctic Research Laboratory Collection ,? August 1948, Point Barrow , Alaska, Beaufort Sea, depth 99.9 m, collector G.E. MacGinitie. ZIRAS 71 /50109, large bifoliate colony, PIBOC Collection , RV Akademik Oparin , 14th Expedition , Stn 15, 12 August 1991, near Kodiak Island , Gulf of Alaska, Pacific Ocean, 58°22.4ʹ N, 150°56.8ʹ W, depth 61 m, Sigsbee trawl, collector A. V. Smirnov GoogleMaps .
Additional material. 71 specimens. IMB Collection (1973) Stns 110/292, 150/385; KIENM Collection (1988) Stns 148, 182, 326, 348, 380, 406; (1991) Stns 219, 226; (1992) Stns 5, 38, 53, 54, 55, 89, 99, 113, 117, 118, 124, 125, 126, 129, 132, 137, 146; (2008) Stn 1–K–1; PIBOC Collection (1991) Stns 14, 16, 17, 58; A. V. Grischenko Collection (1991) Stn 17; KamchatNIRO Collection (2013) Stn 69 (see Appendix 1 for details).
Measurements. NHMUK 1899.5.1.273, locality unknown ( Fig. 3B, C, I View FIGURE 3 ). ZL, 0.80–1.43 (1.03 ± 0.14). ZW, 0.42–0.70 (0.53 ± 0.06). ZD, 0.50–0.55 ( n = 2). OrL, 0.22–0.30 (0.27 ± 0.03). OrW, 0.25–0.37 (0.30 ± 0.03). OeL, 0.27–0.40 (0.34 ± 0.03). OeW, 0.37–0.50 (0.44 ± 0.03). Av(s)L, 0.15–0.30 (0.21 ± 0.04). Av(ad)L, 0.32–0.67 (0.46 ± 0.09). P(m)N, 8–15 (10). P(oe)N, 9–14 (12).
ZIRAS 71/50109, Kodiak Island, Gulf of Alaska, Pacific Ocean ( Fig. 3A, D–F, H, J–M View FIGURE 3 ). ZL, 0.71–1.08 (0.91 ± 0.10). ZW, 0.39–0.63 (0.50 ± 0.05). ZD, 0.47–0.51 ( n = 2). OrL, 0.22–0.31 (0.26 ± 0.02). OrW, 0.22–0.30 (0.26 ± 0.02). OeL, 0.27–0.35 (0.32 ± 0.02). OeW, 0.33–0.50 (0.43 ± 0.04). Av(s)L, 0.14–0.25 (0.19 ± 0.03). Av(ad)L, 0.29–0.45 (0.37 ± 0.04). P(m)N, 8–13 (11). P(oe)N, 10–17 (15) ( n = 20).
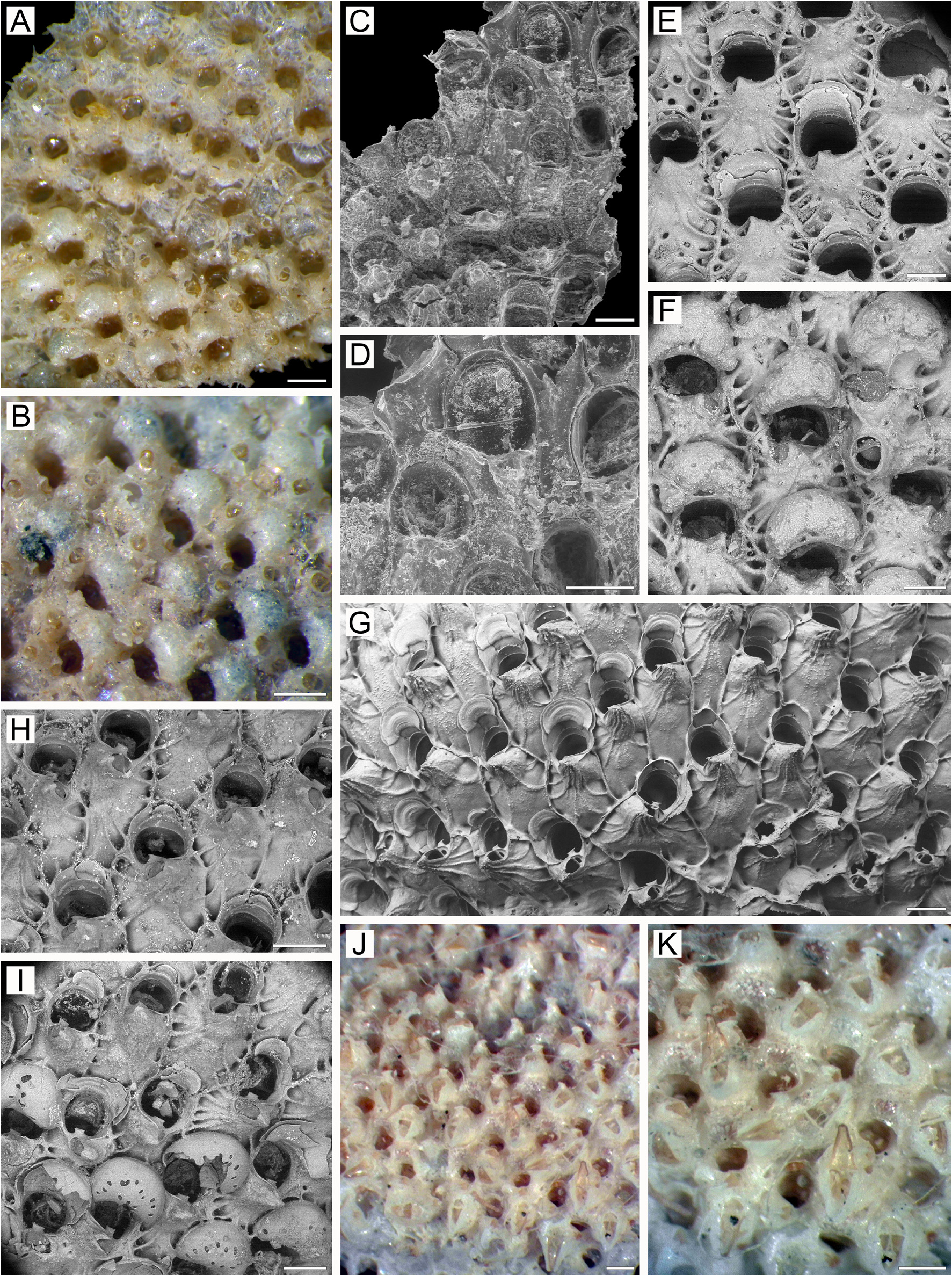
Description. Colonies initially encrusting, multiserial, unilaminar, but rapidly forming extensive bilamellar ( Fig. 3A, M View FIGURE 3 ) ruffled vertical structures attaining up to 70 × 52 mm in size; bright yellow when alive, light yellow when dried. Bilaminate parts of colony reaching thickness up to 1.15 mm. Colonies lacking complete accretion of adjoining layers, with narrow slit-like spaces present between them. Orientation of zooidal rows in apposed layers ( Fig. 3A View FIGURE 3 ) not entirely coinciding, with angles of 5–10° between them. Rates of development of zooids of apposed layers not fully synchronized, with one layer preceding another ( Fig. 3A View FIGURE 3 ). Zooids large, hexagonal to oval, comprising regular straight rows, packed in quincunx; demarcated by lateral and transverse walls, sometimes with fine sutures. Boundaries between zooids clearly visible in young parts of colony but obscured by secondary calcification in older parts ( Fig. 3D–G View FIGURE 3 ).
Frontal shield ( Fig. 3A, D, E, I View FIGURE 3 ) thickened, convex, smooth or with sparse fine granulation in young zooids and densely granular in older parts of colony, with round to elongate-oval and angular areolae along zooidal margins, separated by relatively long, narrow radially arranged interareolar ridges, some of which (distal as well as proximal) connected with cystid of suboral avicularium ( Fig. 3D–F View FIGURE 3 ). Umbonuloid component occupying about 60% of length of frontal shield (58% in one measured zooid), with fine parallel lineation and accretionary banding ( Fig. 3I View FIGURE 3 ). Ring scar discrete ( Fig. 3L View FIGURE 3 ), forming regular boundary between exterior-walled umbonuloid part with exterior-wall planar-spherulitic fabric and extra-umbonuloid part having interior wall microstructure.
Primary orifice ( Fig. 3E, J View FIGURE 3 ) irregularly rounded to oval, often angular; distal and lateral margins formed by upper terminal part of distal transverse wall ( Fig. 3A, D, E View FIGURE 3 ). Distal margin of orifice shallowly rounded, proximal margin concave with anvil-shaped or trapezoidal median lyrula. Condyles absent. Oral spines absent in majority of zooids, but two ephemeral oral spines occasionally evident in marginal zooids.
Secondary orifice ( Fig. 3D–G View FIGURE 3 ) round to oval or irregularly triangular in outline, cormidial, formed proximally by smooth distal part of frontal shield incorporating avicularian cystid. Frontal shield later forming very low, vertical collar-like extension surrounding secondary orifice ( Fig. 3D, E View FIGURE 3 ), especially evident in ovicellate zooids ( Fig. 3F View FIGURE 3 ). Secondary orifice restricted distally and distolaterally by vertical walls of distal and lateral zooids. Outline of secondary orifice often changing during development of secondary calcification, acquiring an irregular oval ( Fig. 3G View FIGURE 3 ) or even roughly triangular shape.
Suboral avicularian cystid relatively small, occupying one-third of zooidal frontal shield or less, conical, strongly elevated, abruptly narrowing terminally, with blunt tip, placed on left or on right side with respect to zooidal orifice; surface finely granular, with 3–5 communication pores ( Fig. 3B–E View FIGURE 3 ); avicularian frontal surface (rostral/postmandibular areas) situated on left or right slope of cystid, usually crossed by zooidal midline, facing obliquely laterally. Rostrum elongate-triangular or oval-like, with blunt apex and small, finely denticulate hook at rostral tip ( Fig. 3B, C View FIGURE 3 ), directed medially or proximomedially upwards. Palate elongate-triangular to semielliptical. Palatal foramen large, conforming to shape of both rostrum and palate, opesia more or less semicircular. Crossbar complete.
Large adventitious avicularium of similar form, developing on frontal shield of older zooids, occupying entire surface of zooid, sometimes extending to marginal area of neighbouring lateral zooid ( Fig. 3G, H View FIGURE 3 ); cystid broad, strongly elevated, with finely granulated surface, frontal surface of avicularium facing distofrontally; rostrum elongate-triangular, blunt, with hooked tip, directed proximolaterally to proximally; palate and palatal foramen elongate-triangular to spatulate, with rounded distal end, opesia semicircular. Crossbar complete. In older parts of colony, adventitious avicularia and thick secondary calcification covering most of frontal surface, strongly changing appearance of zooids (compare Fig. 3D, E, F View FIGURE 3 with Fig. 3G View FIGURE 3 ).
Ovicells cleithral, hyperstomial in young zooids ( Fig. 3F View FIGURE 3 ), becoming subimmersed in older zooids due to secondary calcification arising from daughter and neighbouring zooids and covering ooecium. Further development of secondary calcification resulting in strong immersion, with brood chamber becoming similar to an endozooidal ovicell ( Fig. 3G–H View FIGURE 3 ). Ooecium broader than long, proximal margin slightly concave. Ectooecium initially smooth, with scattered round, oval, or irregular pseudopores. In older zooids, sometimes slightly eroded and partly covered by finely granular secondary calcification, sometimes showing fine sutures demarcating calcification originating from different zooids; some peripheral pseudopores partly occluded by secondary calcification ( Fig. 3G View FIGURE 3 ). Ooecium formed by distal autozooid from slit-like concavity with communication pore at bottom, situated in proximalmost part of frontal shield immediately distal to margin of maternal primary orifice ( Fig. 3D, E View FIGURE 3 ); communication pore leading to canal connecting ooecial and visceral coeloms, opening on underside of frontal shield of distal zooid as straight, slit-like communication pore very close to transverse wall ( Fig. 3I View FIGURE 3 ).
Zooids interconnected by one or two mural pore chambers in each distolateral wall ( Fig. 3M View FIGURE 3 ). Usually, two multiporous septula in basal half of transverse walls ( Fig. 3A View FIGURE 3 ), sometimes with additional individual/paired pores in between. Some transverse walls with one or three septula of various sizes.
Basal surface of zooids ( Fig. 3K View FIGURE 3 ) fully calcified, inflated, with sparse white spots; rare tubular protuberances present ( 0.09–0.28 mm in diameter), narrowing terminally. Boundaries between zooids recognizable basally by deep, slightly meandering incisions.
Ancestrula and early astogeny not observed.
Remarks. Although Lorenz (1886) interpreted Smitt’s (1868a, pl. 28) figures 186–188 as depicting his new species R. costata , and gave his own image (pl. 7, fig. 11), we doubt whether he correctly attributed the specimen for Smitt’s fig. 188 to R. costata .
In the general appearance of zooids and the particular combination of 1) an elevated, conical suboral avicularium and 2) strongly protruding adventitious avicularia that occupy the entire surface of the frontal shield and have an elongate-triangular rostrum with a hooked tip, this species strongly resembles R. cristata ; this resemblance has resulted in many instances of misidentification of both species (see the synonymy for R. cristata below). However, R. costata clearly differs from R. cristata as follows: 1) the proximal border of the primary orifice always has a central lyrula in R. costata , whereas there is only a small median prominence in R. cristata (if an equivalent process develops in the latter species, it is always broad and very shallow); 2) the lateral margins of the secondary orifice are even in R. costata but have tall, symmetrical triangular lappets in R. cristata ; 3) the palate in adventitious avicularia is elongate-triangular to spatulate, with a rounded tip, in R. costata , whereas it is strictly elongate-triangular and acute in R. cristata ; 4) the ridges separating areolae reach only the sides of the suboral avicularian cystid in R. costata but continue to the cystid apex in R. cristata , giving a very characteristic costate pattern to the frontal shield exterior; 5) ovicells are initially prominent, but the ooecium is rapidly overgrown by secondary calcification proceeding from surrounding zooids, leaving only the central area of ectooecium free in R. costata , whereas they remain exposed and not covered by calcification in R. cristata ; 6) colonies of R. costata are initially encrusting, later producing erect parts, whereas those of R. cristata are solely encrusting; and 7) numerous protuberances develop on the basal surface of zooids in R. costata , wheras these protuberances are rare in R. costata .
Canu & Bassler (1928) described Rhamphostomella magnirostris from Cedar Keys, western Florida, Gulf of Mexico (about 29°05.0ʹ N, 83°04.0ʹ W), pointing out the obvious resemblance of their new species to R. costata . Our SEM examination of the syntype specimen of R. magnirostris (USNM 7579) clearly indicated that it is R. costata ( Fig. 31H, I View FIGURE 31 ). One explanation for the presence of a boreal-Arctic species in the tropical Caribbean is that it was introduced, but it is more likely that the specimen was collected in the Arctic/boreal region of North America and subsequently mistakenly labeled.
The Canadian Museum of Nature contains a dried specimen of R. costata from British Columbia identified by O’Donoghue (Cat. No. – CMNI 1988-0286, False Narrows), but unfortunately, we were unable to examine the specimen or images of it.
Ecology. Rhamphostomella costata has been reported over a depth range of 0–308 m, predominantly on hard bottoms (including rocky plateaus, silty rocky platforms, crevices and vertical surfaces), inhabiting rocky substrates such as boulders, blocks, and pebbles). Colonies also encrust bivalve and gastropod mollusc shells, polychaete tubes, hydroids, barnacles and other bryozoans (e.g. Dendrobeania fruticosa , Celleporina nordenskjoldi ) and are occasionally found on flexible substrates such as the thalli of laminarian and red algae. Large, erect parts of colonies of R. costata in turn provide a niche for a diverse epibiotic community, including hydrozoans, spirorbid polychaetes, other bryozoans, ascidians and amphipod and isopod crustaceans.
Distribution. This boreal-Arctic, circumpolar, sublittoral to upper bathyal species is widely distributed in the seas of the Northern Hemisphere. Arctic records include the Barents Sea ( Nordgaard 1896, 1900, 1907a, 1912, 1918; Bidenkap 1900a, 1900b; Waters 1900; Andersson 1902; Norman 1903; Kluge 1906, 1915, 1962, 1975; Kuznetsov 1941; Denisenko 1988, 1990), White Sea ( Kluge 1908 a, 1928; Gostilovskaya 1957, 1978; Grishankov 1995; Ostrovsky 2009, 2013), Kara Sea ( Levinsen 1887; Kluge 1962, 1975; Denisenko 2021), Laptev Sea ( Kluge 1929, 1962, 1975; Gontar 1990), East Siberian Sea ( Nordgaard 1929; Kluge 1929, 1962, 1975; Gontar 1994a, 1994b; Denisenko 2011), Chukchi Sea ( Kluge 1929, 1962, 1975; Denisenko 2008; Denisenko & Kuklinski 2008; Gontar 2010), Point Barrow, Alaska, Beaufort Sea ( Osburn 1955), Canadian Arctic Archipelago ( Nordgaard 1906, 1929; Osburn 1932, 1936), Baffin Bay ( Hansen 1962), Hudson Bay ( Gontar & Denisenko 1989), Labrador ( Osburn 1912b), Davis Strait ( Kluge 1962, 1975; Hansen 1962), western Greenland ( Smitt 1868a; Norman 1906; Kluge 1908b; Osburn 1919, 1936; Denisenko & Blicher 2021), eastern Greenland ( Andersson 1902; Denisenko 2008; Denisenko & Kuklinski 2008; Denisenko & Blicher 2021), Jan Mayen Island ( Lorenz 1886), Franz-Jozef Land ( Denisenko 1990), Spitsbergen ( Gontar et al. 2001; Kuklinski 2002b, 2009; Kuklinski & Bader 2007b; Kuklinski & Taylor 2009) and northern Norway ( Nordgaard 1905, 1918). In the northwestern Atlantic, R. costata is known from the Gulf of St Lawrence ( Hincks 1889; Whiteaves 1901) and the Gulf of Maine southwards to Cape Cod and Woods Hole ( Osburn 1912a, 1933; Powell 1968b; Ryland & Hayward 1991). In the northwestern Pacific, it is known from the Bering Sea, including Chaplin Cape, Chukotsky Cape, St Lawrence Island, Anadyrsky Gulf, Navarin Cape ( Kluge 1961; Grischenko 2002), Korfa Gulf (our data), Litke Strait (our data), Africa Cape, Avacha Gulf ( Kluge 1961; Grischenko 2002; our data), Commander Islands ( Grischenko 1997, 2002); Sea of Okhotsk along the western Kamchatka shelf and slope ( Grischenko 2015; our data), eastern coast of southern Sakhalin Island ( Kluge et al. 1959; Kluge 1961), Aniva Gulf ( Kluge 1961), Shantar Archipelago ( Kluge 1961), Kuril Islands, including Paramushir and Shikotan ( Kluge 1961; Gontar 1980), and South Kuril Strait ( Kluge et al. 1959); Sea of Japan, including Tatar Strait, western shore of the southern Sakhalin Island ( Androsova 1958; Kluge et al. 1959; Kluge 1961), northern Primorje ( Kluge 1961; Tarasova 1983), Hokkaido ( Mawatari 1965; Mawatari & Mawatari 1981). In the northeastern Pacific this species is known from Cook Inlet ( Kessler 1985; Foster 2010) and near Kodiak Island, Gulf of Alaska ( Dick & Ross 1986, 1988; our data) south to Puget Sound ( O’Donoghue & O’Donoghue 1925).
| NHMUK |
Natural History Museum, London |
| T |
Tavera, Department of Geology and Geophysics |
| RV |
Collection of Leptospira Strains |
| V |
Royal British Columbia Museum - Herbarium |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Flustrina |
|
SuperFamily |
Lepralielloidea |
|
Family |
|
|
Genus |
Rhamphostomella costata Lorenz, 1886
| Grischenko, Andrei V., Gordon, Dennis P., Taylor, Paul D., Kuklinski, Piotr, Denisenko, Nina V., Spencer-Jones, Mary E. & Ostrovsky, Andrew N. 2022 |
Rhamphostomella scabra var. costata :
| Nordgaard, O. 1929: 7 |
Rhamphostomella costata :
| Ryland, J. S. & Hayward, P. J. 1991: 39 |
| Dick, M. H. & Ross, J. R. P. 1988: 67 |
| Tarasova, N. A. 1983: 25 |
| Gostilovskaya, M. G. 1978: 226 |
| Mawatari, S. 1965: 619 |
| Kluge, G. A. 1962: 537 |
| Androsova, E. I. 1958: 171 |
| Osburn, R. C. 1912: 244 |
| Nordgaard, O. 1905: 171 |
| Hincks, T. 1889: 426 |
Rhamphostomella costata
| Lorenz, L. von 1886: 12 |
Cellepora scabra : Smitt 1868a , p. 30
| Smitt, F. A. 1868: 30 |