Rhamphostomella scabra ( Fabricius, 1824 )
|
publication ID |
https://doi.org/10.11646/zootaxa.5131.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:CF550031-D6A9-48A3-A953-A1BD40C72F5E |
|
DOI |
https://doi.org/10.5281/zenodo.7628932 |
|
persistent identifier |
https://treatment.plazi.org/id/03892374-0B31-3337-FF73-AE471BFDF92D |
|
treatment provided by |
Plazi |
|
scientific name |
Rhamphostomella scabra ( Fabricius, 1824 ) |
| status |
|
Rhamphostomella scabra ( Fabricius, 1824) View in CoL
( Figs 1 View FIGURE 1 , 25A View FIGURE 25 , 30A View FIGURE 30 , 31A, B, E, F View FIGURE 31 )
Eschara scabra Fabricius, 1824 , pp. 29, 30, tab. 1, figs 1–3.
Cellepora scabra: Smitt 1868a, p. 30 (part), pl. 28, figs 183‒185, (?)188; 1868b, pp. 484, 485.
Rhamphostomella scabra: Lorenz 1886, p. 11 View in CoL , 12; Nordgaard 1905, p. 171, pl. 5, figs 8–11; Kluge 1955, p. 108, tab. 23, fig. 5; 1962, p. 536, fig. 374; 1975, p. 653, fig. 374; Gostilovskaya 1978, p. 225, fig. 141; Gordon & Grischenko 1994, p. 64, figs 7–12.
Discopora scabra: Nordgaard 1918, p. 77 .
Rhamphostomella sollers Canu & Bassler, 1929, p. 353 View in CoL , pl. 43, figs 4–10.
Additional references. Rhamphostomella scabra: Nordgaard 1906, p. 30 View in CoL , 41; Kluge 1907, p. 196; 1908b, p. 553; 1929, p. 21; 1953, p. 178; 1961, p. 141; Osburn 1936, p. 542; Gostilovskaya 1957, p. 455; 1964, p. 219; Androsova 1977, p. 202; Kluge et al. 1959, p. 213; Hansen 1962, p. 41; Powell 1968a, p. 2312; Gontar 1980, p. 13; 1990, p. 132; 1992, p. 194; 1993b, p. 202; 1994a, p. 145; 1996, p. 46; 2010, p. 153; 2013, p. 48; Mawatari & Mawatari 1981, p. 55; Tarasova 1983, p. 26; Denisenko 1988, p. 13; 1990, p. 39; 2008, p. 188; 2011, p. 14; 2013, p. 184; Gontar & Denisenko 1989, p. 354; Grishankov 1995, p. 48; Kubanin 1997, p. 123; Grischenko 1997, p. 174; 2002, p. 114; 2015, p. 40; Grischenko et al. 1999, p. 112; Gontar et al. 2001, p. 195; Shunatova & Ostrovsky 2001, p. 118; Kuklinski 2002a, p. 181; 2002b, p. 203; 2009, p. 228; Grischenko & Mawatari 2002, p. 129; Kuklinski & Bader 2007a, p. 713; 2007b, p. 840; Denisenko & Kuklinski 2008, p. 48.
Rhamphostomella sollers: Kataoka 1957, p. 146 View in CoL ; Sakagami et al. 1980, p. 330.
Material examined. Neotype: SMNH-Type-9304, one colony fragment, 1837, Hammerfest , Norway, North Atlantic Ocean, depth 73–110 m, collector S. Lovén.
SMNH-127473, one colony fragment, Norway, North Atlantic Ocean , collector S. Lovén. GoogleMaps SMNH-128630, one colony on polychaete tube, Jenissej Expedition , Marine Stn 37, 1876, Kara Sea , 74°30.0ʹ N, 65°35.0ʹ E, depth 64 m. GoogleMaps ZIRAS 10 , two colony fragments, RV Sibiriakov, Stn 34, 14 September 1933, in front of Maria Pronchishcheva Bay , east side of Taymyr Peninsula, Laptev Sea, 75°38.0ʹ N, 112°58.0ʹЕ, bottom trawl, collector G.I. Gorbunov. GoogleMaps USNM 208837 About USNM , two colony fragments detached from shell of bivalve mollusc, Arctic Research Laboratory Collection,? August 1948, Point Barrow, Alaska, Beaufort Sea, collector G.E. MacGinitie. GoogleMaps ZIRAS 93/50106 , five colony fragments, KIENM Collection, Stn 215, 30 July 1991, profile: Cape Vkhodnoy Reef– Toporkov Island , coastal waters of Bering Island , Pacific Ocean, 55°11.9ʹ N, 165°56.9ʹ E, depth 15 m, SCUBA, collector D.D. Danilin. GoogleMaps ZIRAS 94 /50107, one colony fragment, PIBOC Collection, RV Akademik Oparin, 14th Expedition, Stn 89, 10 September 1991, coastal waters of the Lesser Kuril Ridge, Pacific Ocean, 43°40.5ʹ N, 146°45.2ʹ E, depth 102 m, Sigsbee trawl, collector A. V. Smirnov. GoogleMaps ZIRAS 95/50108 , two colony fragments detached from broken shells of bivalve mollusc Chlamys sp. , MFRT Rodino, 12 September 1992, about 32 km from Cape Hayryuzova, western Kamchatka shelf, Sea of Okhotsk, 57°36.2ʹ N, 156°09.0ʹ E, depth 78–81 m, crab trap, collector A.V. Grischenko. GoogleMaps MIMB 2/50395 , three colony fragments, IMB Collection, RV Atma, Stn 232/646, 3 August 1976, south-eastern coastal waters off Moneron Island, Sea of Japan, depth 80 m, Sigsbee trawl, collector V.I. Lukin GoogleMaps .
Additional material. 40 specimens. IMB Collection (1972) Stn 32/99; (1973) Stns 215/536, 224/582; KIENM Collection (1988) Stns 86, 182; (1991) Stns 215, 220; (1992) Stns 25, 30, 38, 54, 97, 128; PIBOC Collection (1991) Stn 91; A. V. Grischenko Collection (1991) Stn 10; KamchatNIRO Collection (2013) Stns 63, 82 (see Appendix 1 for details) .
Measurements. USNM 208837, Beaufort Sea ( Fig. 1H, J, L View FIGURE 1 ). ZL, 0.92–1.59 (1.15 ± 0.17). ZW, 0.55–0.90 (0.72 ± 0.09). ZD, 0.62–0.82 ( n = 2). OrL, 0.25–0.35 (0.30 ± 0.03). OrW, 0.32–0.40 (0.37 ± 0.02). OeL, 0.32–0.37 (0.35 ± 0.03) ( n = 4). OeW, 0.43–0.52 (0.48 ± 0.04) ( n = 4). Av(s)L, 0.14–0.22 (0.18 ± 0.02). Av(ad)L, 0.20–0.35 (0.29 ± 0.04). P(m)N, 15–22 (17). P(oe)N, 9–18 (14) ( n = 4).
ZIRAS 93/50106, Bering Island, Commander Islands, Pacific Ocean ( Figs 1B, E–G, I, K, M View FIGURE 1 , 30A View FIGURE 30 ). ZL, 0.68– 1.15 (0.82 ± 0.09). ZW, 0.42–0.58 (0.49 ± 0.05). ZD, 0.60–0.67 ( n = 2). OrL, 0.20–0.25 (0.23 ± 0.02). OrW, 0.20– 0.30 (0.26 ± 0.03). OeL, 0.25–0.33 (0.28 ± 0.02) ( n = 25). OeW, 0.35–0.48 (0.41 ± 0.03) ( n = 25). Av(s)L, 0.10–0.18 (0.13 ± 0.02). Av(ad)L, 0.15–0.26 (0.20 ± 0.03). P(m)N, 8–17 (12). P(oe)N, 3–12 (6).
MIMB 2/50395, Moneron Island, Sea of Japan ( Fig. 1D View FIGURE 1 ). ZL, 0.82–1.23 (1.05 ± 0.09). ZW, 0.60–0.93 (0.76 ± 0.08). ZD, 0.63–0.74 ( n = 2). OrL, 0.27–0.34 (0.31 ± 0.02). OrW, 0.29–0.38 (0.35 ± 0.02). Av(s)L, 0.16–0.24 (0.20 ± 0.02). Av(ad)L, 0.25–0.37 (0.30 ± 0.03). P(m)N, 14–20 (17).
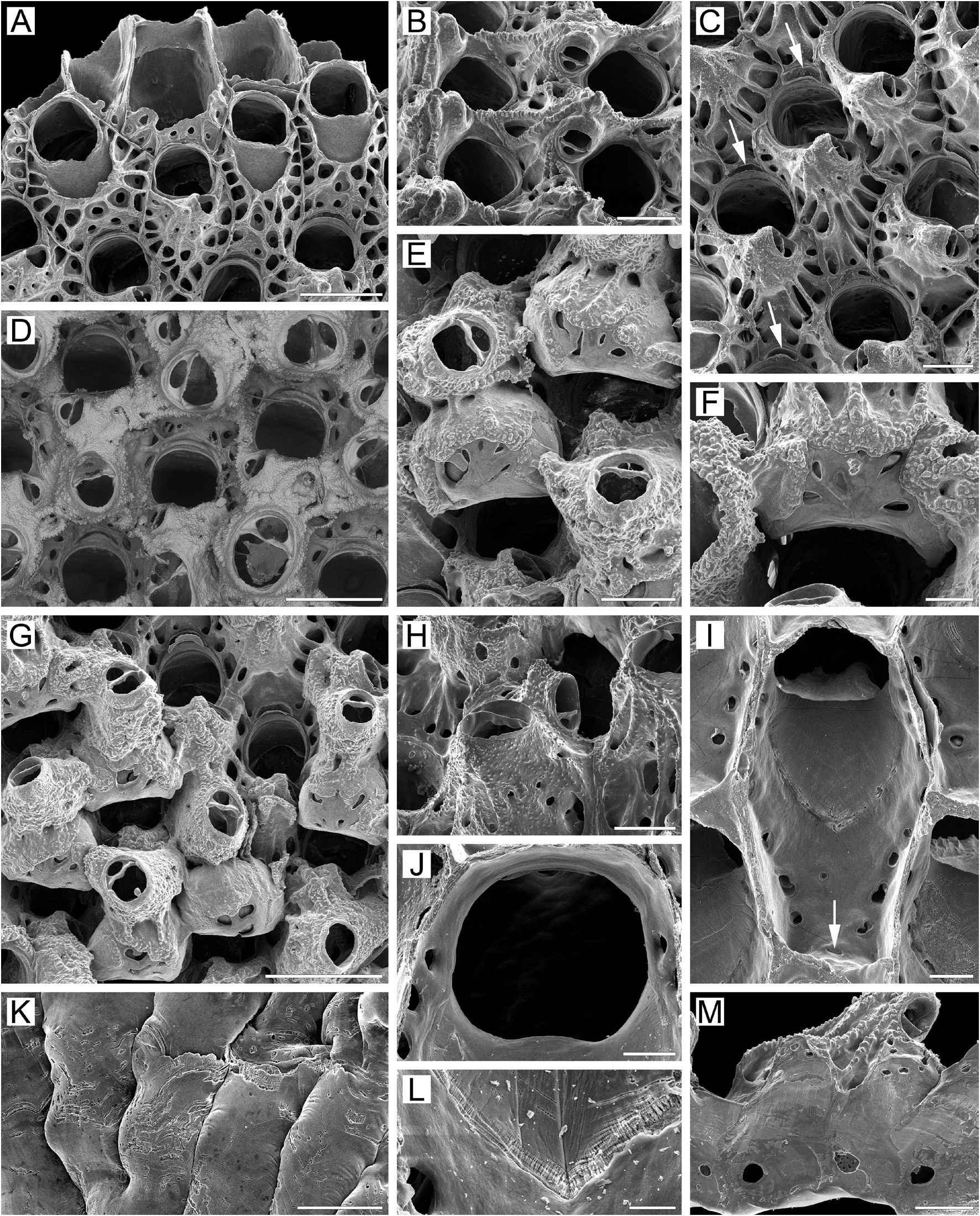
Description. Colonies encrusting, multiserial, unilaminar ( Fig. 1A View FIGURE 1 ), attaining up to 23 mm in any one direction, occasionally forming small erect bilamellar expansions and frills, orange to bright yellow when alive, light yellow when dry. Zooids large, broadly hexagonal to oval, arranged in regular rows in checkered pattern, demarcated by fine undulating sutures between vertical lateral walls in young parts of colony ( Fig. 1A, C View FIGURE 1 ); these sutures occluded by secondary calcification in older parts of colony ( Fig. 1D, G View FIGURE 1 ).
Frontal shield ( Fig. 1A, C, I View FIGURE 1 ) umbonuloid, convex, with finely granulated surface in young zooids and with coarse granulation in older zooids; with numerous areolae separated by long, narrow, radially arranged interareolar ridges. In young zooids, deep pits around areolae irregularly oval to triangular, trapezoid or quadrate, occurring in one marginal row laterally and in 2–3 rows proximally (examination of underside of frontal shield showed that centrally placed “holes” are not pseudopores) ( Fig. 1A View FIGURE 1 ). In older zooids, secondary calcification of frontal shield resulting in fusion of proximal pits around areolae in one proximal row; these pits also becoming elongate, as do interareolar ridges connected with cystid of suboral avicularium that sometimes continue to apex to form small, pointed bulge ( Fig. 1B–D, G View FIGURE 1 ). Areolar pits surrounding areolar openings (for convenience, termed areolae throughout the text) reduced in size in oldest zooids owing to thickening of frontal shield by secondary calcification. Umbonuloid component occupying about 50% of length of frontal shield, with fine, parallel lineation and accretionary banding. Ring scar discrete ( Fig. 1L View FIGURE 1 ), forming regular boundary between umbonuloid exterior wall and extra-umbonuloid interior wall microstructure.
Primary orifice ( Fig. 1A, J View FIGURE 1 ) roundly quadrate or bell-shaped to transversely oval; distal and lateral margins formed by upper terminal part of distal transverse wall, sometimes bearing narrow shelf distally and forming illdefined condyles laterally ( Fig. 1A, D View FIGURE 1 ); condyles not present in all zooids ( Fig. 1A View FIGURE 1 ). Distal margin of orifice round, proximal margin straight or with very weak median prominence; proximolateral corners broadly rounded. Oral spines absent in “mature” zooids; two ephemeral spines can be seen at distal margin of primary orifice in some young zooids at colony periphery.
Secondary orifice ( Fig. 1C, D, G View FIGURE 1 ) irregularly oval, cormidial, restricted proximally by distal area of frontal shield incorporating avicularian cystid. Distally and distolaterally, secondary orifice formed by elevated vertical walls of distal and lateral zooids.
Suboral avicularium with cystid not very large, occupying distal one-third to half of frontal shield, mostly situated centrally in respect to zooidal orifice ( Fig. 1A‒C View FIGURE 1 ); cystid conical, elevated (although less prominent in old zooids, Fig. 1D View FIGURE 1 ), with finely granulated surface; 2–5 small communication pores visible in wall of young avicularium ( Fig. 1A, C View FIGURE 1 ). Frontal surface of avicularium (including rostral and postmandibular areas) situated on left or right slope of avicularian cystid, out of zooidal midline, facing distolaterally to laterally ( Fig. 1A, C View FIGURE 1 ). Rostrum semioval, blunt ( Fig. 1B–E View FIGURE 1 ), directed obliquely upwards. Palate short, semioval to semielliptical; in some zooids in same plane as postmandibular area ( Fig. 1C–E, F, H View FIGURE 1 ), whereas in others there is right angle between them ( Fig. 1A, D View FIGURE 1 ). Palatal foramen repeats shape of palate, semioval opesia (postmandibular area) oval or ellipsoidal. Crossbar complete, with small, low ligula.
Large adventitious avicularia of two size categories occupying proximal half of frontal shield in older zooids ( Fig. 1D, E, G, H View FIGURE 1 ). Avicularian cystid broad, elevated, with finely granulated surface and 6–8 proximolateral communication pores ( Fig. 1H View FIGURE 1 ). Avicularian frontal surface facing obliquely upwards. Rostrum broadly oval, blunt, directed medially upwards. Palate short, broadly semioval. Palatal foramen repeating shape of palate, semioval, opesia elliptical. Crossbar complete, often with low ligula. In older parts of colony, adventitious avicularia occupying most of frontal surface, rendering frontal shields scarcely visible ( Fig. 1D, G View FIGURE 1 ).
Ovicell initially hyperstomial, but ooecium rapidly becoming covered by secondary calcification expanding from frontal shields of distal and distolateral zooids. Borders between calcification from different sources appearing as fine, meandering sutures ( Fig. 1E–G View FIGURE 1 ). Secondary calcification granular, covering from half to two-thirds of ooecium, so ovicells becoming subimmersed and even appearing endozooidal ( Fig. 1F View FIGURE 1 ). Ooecium formed by distal autozooid around shallow crescentic concavity with communication pore at bottom, situated in most proximal part of frontal shield just immediate to distal margin of maternal primary orifice ( Fig. 1A, C, D, G View FIGURE 1 ). Pore leading to communication canal connecting ooecial and visceral coeloms and forming straight, slit-like ooecial communication pore on underside of frontal shield of distal zooid, very close to transverse wall ( Fig. 1I View FIGURE 1 ). Ooecium spherical, broader than long, with wide, straight or slightly concave proximal margin ( Fig. 1E–G View FIGURE 1 ). Ectooecium smooth, with oval, drop-like or slit-like pseudopores sometimes arranged in radial pattern. Ooecial base surrounded by round, oval or elongated areolae, separated by short, narrow ridges.
Zooids interconnected by one mural pore chamber in each distolateral wall (corresponding to large opening present in proximolateral wall) ( Fig. 1M View FIGURE 1 ). Communication pores spread through basal half of transverse wall either as wide horizontal “band” or forming 1–4 groups (multiporous septula) of varying size, outline and pore density. Both patterns sometimes composed of random individual pores, and wide septula (pore “band”) can be composed of small pore groups.
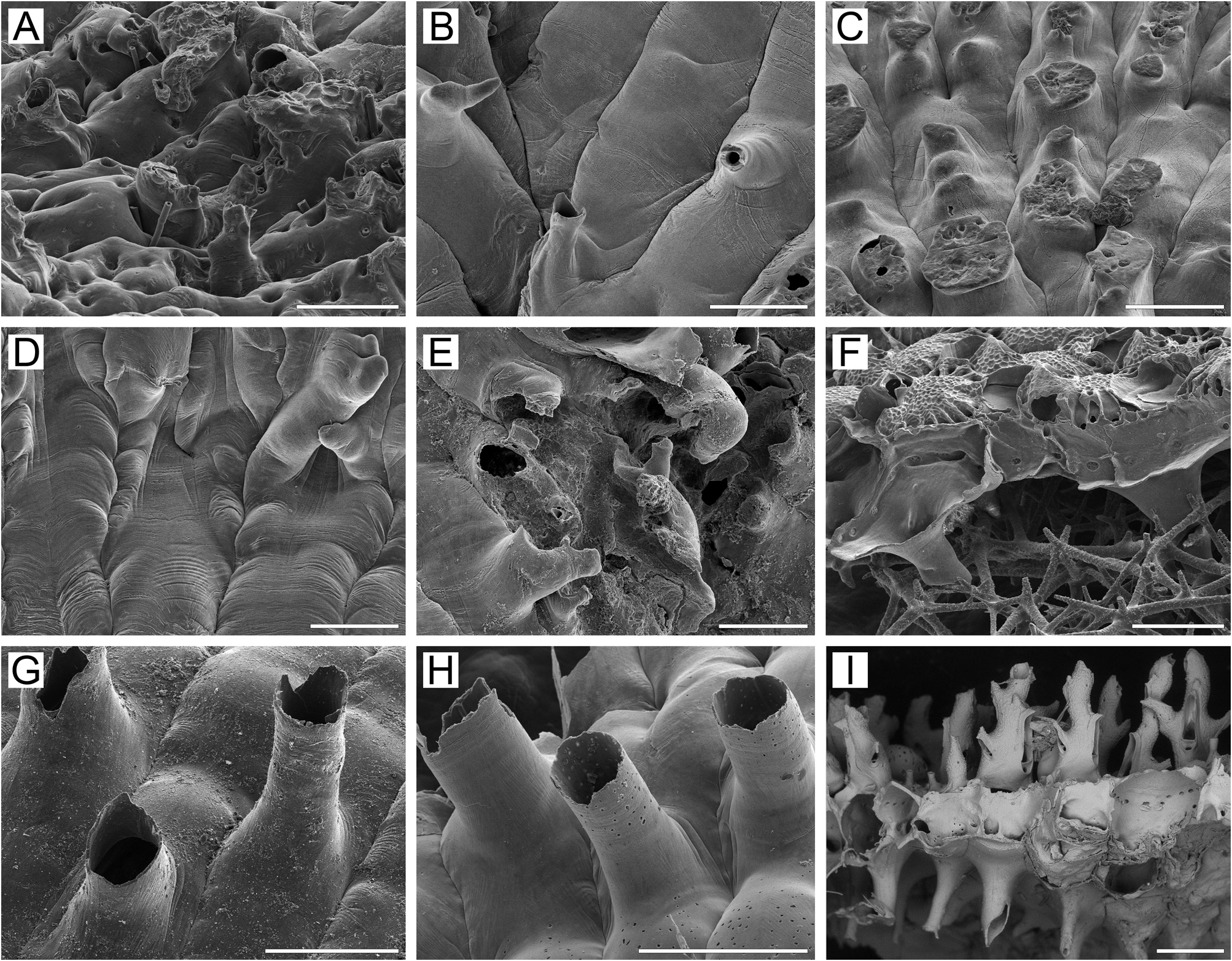
Basal surface of zooids ( Fig. 1K View FIGURE 1 ) fully calcified, inflated. Numerous white spots (presumably lightly calcified areas) visible in semitransparent basal wall under light microscope. Numerous tubular protuberances ( 0.11–0.27 mm in diameter) present on basal surface of marginal colony area, beneath growing edge ( Fig. 30A View FIGURE 30 ). Boundaries between zooids recognizable basally by deep meandering incisions.
Ancestrula ( Fig. 25A View FIGURE 25 ) tatiform, basal outline irregularly circular; opesia circular, with nine basally jointed periopesial spines evenly distributed around opesial margin; budding triplet of periancestrular zooids distally and distolaterally; periancestrular zooids similar to but smaller than subsequent zooids, with three to six ephemeral oral spines around distal margin of orifice.
Remarks. There has been some confusion concerning the type species of Rhamphostomella . Following the suggestion of Smitt (1868a, b), the first information on this cheilostome was provided by Fabricius (1780, p. 433, No 437), who described a specimen from Greenland as Millepora reticulata Linnaeus. Fabricius himself, however, later admitted his mistake, realizing that his specimen belonged to “Coral-Barkene (Escharas)” ( Fabricius 1824, pp. 29, 30). He named it Eschara scabra adding to his description three very schematic drawings all probably showing the same colony fragment under various low magnifications (tab. 1, figs 1‒3). Smitt (1868a, p. 181), based on specimens collected during two different expeditions, redescribed this species as Cellepora scabra , indicating that this “form for the most part seems to have been the basis for Fabricius’s description”. This suggestion remains unconfirmed, since Fabricius’s specimen was supposedly lost; although some of his specimens are kept in the collections of the Natural History Museum of Denmark, University of Copenhagen, Fabricius’s specimen is not mentioned in the museum’s catalogues for either the bryozoan or cnidarian collection (C. Nielsen, pers. comm., 2020).
In the same paper Smitt (1868a) described a new species, C. plicata , stressing that it is similar to C. scabra . He also mentioned both species R. scabra and R. plicata (as Cellepora ) in his large taxonomic work published the same year ( Smitt 1868b).
When Lorenz (1886) introduced the genus Rhamphostomella , he attributed six species to it, two of them new (see Remarks for R. plicata for details). Norman (1903, p. 125) chose “ Ramphostomella scabra (Fabricius) , Smitt”, as the “ Type ”, but uncertainty remains over the identity of Fabricius’s species (see above), reflected in Lorenz’s citation of the species in his synonymy as “ Eschara scabra Fabricius , (teste Smitt)”. Smitt (1868a, pl. 28, figs 183– 185,?188) presented some fairly good drawings of what he referred to as Cellepora scabra . Specimens illustrated in Smitt’s figures 183–185 were interpreted by Lorenz on the basis of a range of specimens from Jan Mayen as depicting R. scabra , and figs 186–188 [? Cellepora scabra Fabricius : Smitt, ex parte] as depicting the new species R. costata Lorenz, 1886 , distinguished especially by the shape of the avicularian mandibles and the generally lyrulate orifice. Levinsen (1909), taking into account the variation exhibited by these nominal species, saw no distinction between them. On the other hand, Hincks (1889), Whiteaves (1901), Norman (1903), Nordgaard (1905, 1906), Osburn (1952), Androsova (1958), Kluge (1962) and Powell (1968a) accepted two species, as we do here. Canu & Bassler (1917), evidently overlooking Norman’s (1903) indication of the type species, selected R. costata as the type. While acknowledging Canu & Bassler’s (1917) oversight, Harmer (1957) nevertheless argued that R. costata should stand in preference to R. scabra , “the trivial name of which belongs to a species insufficiently described by Fabricius.” Harmer’s argument must be rejected ( Hayward 1975), but the conspecificity of Fabricius’s and Smitt’s material remained questionable until now.
Smitt’s (1868a) figures 183–185 (and possibly 188) illustrate R. scabra as the species accepted by the most authors. To preclude further confusion, we have selected a neotype for this species from among specimens of Smitt collected in the North Atlantic ( Norway), kept at the Swedish Museum of Natural History (for additional details and discussion, see Gordon & Grischenko 1994).
Kluge (1961) reported a new subspecies, Rhamphostomella scabra orientalis , from the intertidal zone of Bering Island, the Commander Islands, and Kronotsky Gulf, eastern Kamchatka. Although these records were not accompanied by a description or illustrations, Grischenko (1997, 2002) and Denisenko (2013) reported R. scabra orientalis . Our examination of the type material of R. scabra orientalis (ZIRAS 1/8801) showed that Kluge’s (1961) subspecies was based on a misidentification of Desmacystis sandalia ( Robertson, 1900) , endemic to the North Pacific ( Fig. 31C, D View FIGURE 31 ). Rhamphostomella scabra orientalis is thus a junior synonym of D. sandalia .
Canu & Bassler (1929) described a new species, Rhamphostomella sollers , based on material collected by the RV Albatross from Stn D. 4807 near Cape Tsiuka ( 41°36.1ʹ N, 140°36.0ʹ E), Sangar Strait, Sea of Japan. Our SEM examination of the syntype (USNM 8136) indicates that it is synonymous with R. scabra ( Fig. 31E, F View FIGURE 31 ).
In several cases, R. scabra was apparently misidentified. For example, Androsova’s (1958) material identified as R. scabra is very likely what we describe herein as R. microavicularia n. sp.. Records of R. scabra in particular regional studies in the Far East ( Kluge et al. 1959; Kluge 1961; Gontar 1980, 1993b; Mawatari & Mawatari 1981; Denisenko 2013) might also need to be reexamined, since the material involved could have been confused with other, similar species (discussed in Remarks sections for particular species below).
Ecology. Rhamphostomella scabra has been reported from depths of 0–460 m on various bottom types / substrates, including boulders, pebbles, gravel, sand and silt. In addition to rocky surfaces, this species inhabits the shells of gastropod and bivalve molluscs, the tubes of serpulid and spirorbid polychaetes, and the surfaces of sponges and ascidians.
Distribution. R. scabra is a boreal-Arctic, circumpolar, sublittoral to upper bathyal species, widely distributed in the seas of the Northern Hemisphere. In the Arctic, it has been recorded from the Barents Sea ( Smitt 1868 a, 1878 a, 1878b; Bidenkap 1900a; Andersson 1902; Nordgaard 1905; Kuznetsov 1941; Kluge 1962, 1975; Denisenko 1988, 1990), White Sea ( Smitt 1878a; Kluge 1907, 1962, 1975; Gostilovskaya 1957, 1978; Grishankov 1995; Shunatova & Ostrovsky 2001), Kara Sea ( Smitt 1878b; Levinsen 1887; Nordgaard 1912; Kluge 1929, 1962, 1975; Denisenko 2021), Laptev Sea ( Kluge 1929, 1962, 1975; Gontar 1990, 1996), East-Siberian Sea ( Kluge 1962, 1975; Gontar 1994a; Denisenko 2011), Chukchi Sea ( Kluge 1962, 1975; Denisenko 2008; Gontar 2010), Point Barrow, Alaska, Beaufort Sea ( MacGinitie 1955), Canadian Arctic Archipelago ( Nordgaard 1906; Osburn 1936), Baffin Bay ( Hansen 1962), Davis Strait ( Hansen 1962; Kluge 1962, 1975), Labrador ( Gontar & Denisenko 1989), western Greenland (Henning 1896; Norman 1906; Kluge 1908b; Levinsen 1914; Osburn 1919; Denisenko & Blicher 2021), eastern Greenland ( Andersson 1902; Levinsen 1916; Kuklinski & Bader 2007a, 2007b), Jan Mayen Island ( Lorenz 1886), Spitsbergen ( Gontar et al. 2001; Kuklinski 2002a, 2002b, 2009; Kuklinski & Bader 2007b; Denisenko & Blicher 2021), Franz-Jozef Land ( Denisenko 1990), Lofoten Islands ( Nordgaard 1918), and northern Norway ( Nordgaard 1905). In the northwestern Atlantic, R. scabra extends along the east coast of North America from the Gulf of St Lawrence to the Gulf of Maine ( Whiteaves 1901). In the northwestern Pacific, it has been reported from the Bering Sea in Provideniya Bay, Kamchatskiy Gulf, Kronotsky Gulf and Avacha Gulf ( Kluge 1961; Kubanin 1997; Grischenko 2002; Gontar 2013; our data), Commander Islands ( Kluge 1961; Grischenko 1997, 2002; Kubanin 1997); Sea of Okhotsk, including western Kamchatka shelf and slope ( Grischenko et al. 1999; Grischenko 2015; Grischenko & Mawatari 2002; our data), eastern coastal waters of southern Sakhalin Island ( Kluge et al. 1959; Kubanin 1997), Shantar Archipelago ( Kluge 1961; Kubanin 1997), along Kuril Islands, including Paramushir, Makanrushi, Onekotan, Raschua, Simushir, Urup, Kunashir, Shikotan ( Kluge 1961; Gontar 1980, 1993b), and South Kuril Strait ( Kluge et al. 1959; Gontar 1980); Sea of Japan, along the western shore of southern Sakhalin Island ( Androsova 1958; Kluge 1961; Kluge et al. 1959), Primorye ( Kluge 1961; Tarasova 1983), Moneron Island (our data), Hokkaido ( Mawatari & Mawatari 1981) and Sangar Strait ( Canu & Bassler 1929). We stress here that existing uncertainties in some records mentioned above as well as overlaps of distribution areas of similar species should be considered during future research.
| ZIRAS |
ZIRAS |
| USNM |
USA, Washington D.C., National Museum of Natural History, [formerly, United States National Museum] |
| KIENM |
KIENM |
| PIBOC |
PIBOC |
| MIMB |
MIMB |
| IMB |
IMB |
| RV |
Collection of Leptospira Strains |
| V |
Royal British Columbia Museum - Herbarium |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Flustrina |
|
SuperFamily |
Lepralielloidea |
|
Family |
|
|
Genus |
Rhamphostomella scabra ( Fabricius, 1824 )
| Grischenko, Andrei V., Gordon, Dennis P., Taylor, Paul D., Kuklinski, Piotr, Denisenko, Nina V., Spencer-Jones, Mary E. & Ostrovsky, Andrew N. 2022 |
Rhamphostomella sollers : Kataoka 1957 , p. 146
| Sakagami, S. & Arakawa, S. & Hayami, T. 1980: 330 |
| Kataoka, J. 1957: 146 |
Rhamphostomella sollers
| Canu, F. & Bassler, R. S. 1929: 353 |
Discopora scabra :
| Nordgaard, O. 1918: 77 |
Rhamphostomella scabra :
| Gordon, D. P. & Grischenko, A. V. 1994: 64 |
| Gostilovskaya, M. G. 1978: 225 |
| Kluge, G. A. 1955: 108 |
| Nordgaard, O. 1905: 171 |
| Lorenz, L. von 1886: 11 |
Cellepora scabra : Smitt 1868a , p. 30
| Smitt, F. A. 1868: 30 |