Petrocephalus tanensis ( Whitehead and Greenwood, 1959 )
|
publication ID |
https://doi.org/ 10.1080/00222933.2012.708452 |
|
DOI |
https://doi.org/10.5281/zenodo.10526944 |
|
persistent identifier |
https://treatment.plazi.org/id/038087B4-FFA6-FFD8-A29D-F9D8649730B9 |
|
treatment provided by |
Carolina |
|
scientific name |
Petrocephalus tanensis ( Whitehead and Greenwood, 1959 ) |
| status |
|
Petrocephalus tanensis ( Whitehead and Greenwood, 1959) View in CoL , elevated to species rank
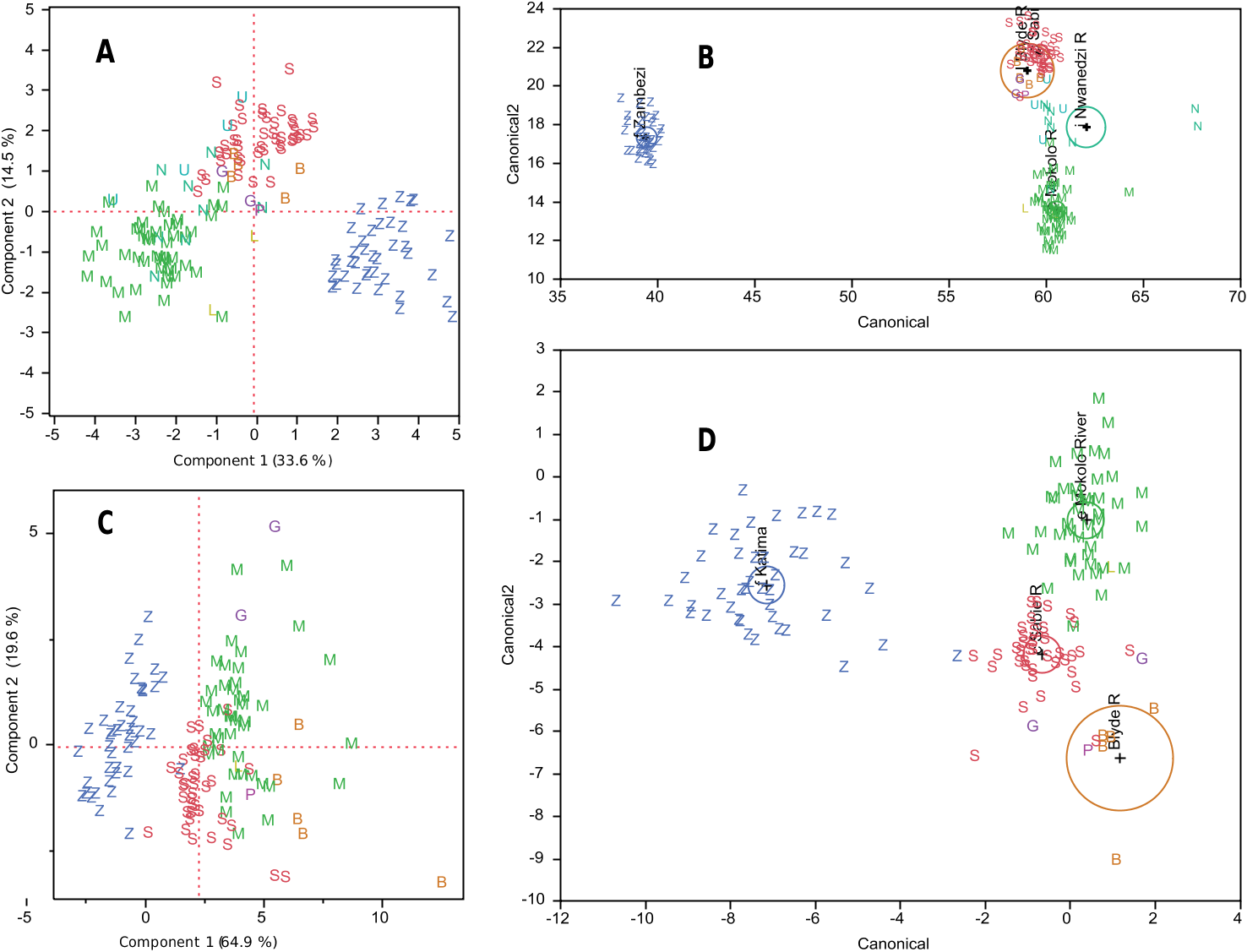
( Figure 2, nos 10, 10a; online Figure 13 View Figure 13 )
Petrocephalus catostoma tanensis Whitehead and Greenwood, 1959: 286 View in CoL . Tanachurchill, Seegers et al. 2003: 29.
Type specimens. Holotype: BMNH 1963.11 .29.1 collected at Kenya, Lower Tana River, Garsen (a settlement in Kenya’s Coast Province ) . Paratypes: BMNH 1963.11.29.2–8 (eight specimens). Studied .
Type locality. Garsen , Tana River, Kenya .
Diagnosis. Distance dorsal fin origin to end of caudal peduncle, pD, mean 0.448 (range 0.427 –0.472) of SL; dorsal fin length, LD, mean 0.192 (range 0.167 –0.222) of SL; number of dorsal fin rays, nD, median 24 (range 22–27); body depth, BD, mean 0.299 (range 0.257 –0.356 of SL; anal fin length, LA, mean 0.239 (range 0.214 –0.257) of SL; depth of caudal peduncle, CPD, mean 0.346 (range 0.291 –0.439) of CPL, length of caudal peduncle; predorsal length, PDL, mean 0.612 (range 0.574 –0.645) of SL; head length, HL, mean 32.9 (range 27.3–42.8) times Na, distance between the pair of nares of one side. EOD characteristics at 25 ◦ C and “2% threshold criterion” (see Material and methods): peak amplitude of P2 phase, P2amp, in males, mean 0.534 (range 0.435–0.69) of P1amp, peak amplitude of P1 phase; in females, mean 0.375 (range 0.021–0.74) of P1amp; duration of P2 phase, P2dur, mean 256 µs (range 105–510 µs); duration of P1 phase, P1dur, mean 130 µs (range 95–198 µs); duration of N phase, Ndur, 46 µs (range 31–191 µs), negative peak amplitude (absolute value) of N phase, Namp, mean – 3.1 (range – 2.382 to – 3.748) of P1amp.
Description. Body oval shape, dorsally deep and rounded, ventrally rather flat ( Figure 2, no. 10). Head broadly rounded with a small ventrally positioned subterminal mouth, situated ventral to the eye; head and body dorsolaterally compressed. Dorsal fin (a) origin situated almost two-thirds of standard length from snout, (b) obliquely orientated, anteriorly higher and posteriorly lower, (c) distal margin crescentic with anterior two or three rays longer than posterior rays, and (d) number of rays 22 (n = 1), 23 (n = 12), 24 (n = 19), 25 (n = 18), 26 (n = 2). Anal fin (a) longer than dorsal fin, (b) opposite dorsal fin with slightly more anterior origin, (c) obliquely orientated, anteriorly lower and posteriorly higher, (d) anterior 10 or so rays longer than posterior ones, especially in males where they also appear stronger, (e) margin broadly rounded, (f) rays posterior to first 10 with distal margin straight, (g) number of rays 26 (n = 1), 27 (n = 16), 28 (n = 24), 29 (n = 13). Forked tail fin with rounded lobes. Scales cycloid with reticulate striae, scales extending anteriorly to operculum and pectoral fins (beyond pelvics). Scales in lateral series, 36 (n = 12), 37 (n = 14), 38 (n = 1). Scales on caudal peduncle circumference, 12 (n = 37), 13 (n = 2), 14 (n = 14). Caudal peduncle slender, subcylindrical entire length, usually 22.3% (19.3–24.7%) of SL ( Table 1). Electric organ discharge a triphasic pulse with strong head-positive phase P1 followed by head-negative main phase N, and weaker headpositive P2 phase ( Figure 10 View Figure 10 ); P2 phase stronger in males than in females of same size. Pulse duration, median 403 (312–735) µs in females (n = 15), and shorter median, 307 (276–391) µs, in males (n = 1 5); 25 ◦ C, 2% threshold criterion. Males with kink in anal fin base which is absent in juveniles and females where the anal fin base is straight.
Colour in preservation. Light ochre.
Colour in life. Grey-silver, underside lighter, paired fins light and transparent. Ecology. Despite the dry season, the terminal section of the Tana River was a strongflowing river with steep and high borders accompanied by gallery forest. The water was muddy brown. Riparian reed vegetation not very prominent.
Distribution. “From Hola, Wema and Garsen, Tana River. Confined to the Tana River and probably not occurring above Garissa (600 feet above sea level)” ( Whitehead and Greenwood 1959); “apparently missing from the Athi” ( Whitehead 1962). Garissa is about 250 km from the Indian Ocean, and the other localities within 130 km or less.
Etymology. Not explained by Whitehead and Greenwood (1959) but clearly thought to be an endemic species to the Tana River.
Remarks. Longest pD among all samples, very long LD, very high nD, and high values for BD, LA, CPD while those for PDL and Na low. EOD distinguished by very strong Namp (modal 3.17 times P1amp), strong P2amp (modal 0.47 times P1amp), and very short Ndur (modal 37 µs at 25 ◦ C). EOD with strongest Namp in spite of shortest Ndur and smallest Narea, short P1dur, P1Nsep shortest, P1P2 shortest, NP2 shortest.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Petrocephalus tanensis ( Whitehead and Greenwood, 1959 )
| Kramer, Bernd, Bills, Roger, Skelton, Paul & Wink, Michael 2012 |
Petrocephalus catostoma tanensis
| Seegers L & De Vos & Okeyo DO 2003: 29 |
| Whitehead PJ & Greenwood PH 1959: 286 |