Cystisoma GuérinMéneville
|
publication ID |
https://doi.org/ 10.5281/zenodo.156376 |
|
DOI |
https://doi.org/10.5281/zenodo.6276428 |
|
persistent identifier |
https://treatment.plazi.org/id/013487FF-B723-FFFC-FEE9-D70AFAF59E60 |
|
treatment provided by |
Plazi |
|
scientific name |
Cystisoma GuérinMéneville |
| status |
|
Genus Cystisoma GuérinMéneville View in CoL
Cystisoma GuérinMéneville, 1842: 215 View in CoL . – Dana 1852: 315. Dana 1853: 981 & 1442. Willemöes Suhm, 1875: 24. Stebbing 1888: 1318. Barnard 1916: 286. Barnard 1932: 268. Pirlot 1938: 364. Pirlot 1939: 33. Bowman & Gruner 1973: 26. Vinogradov et al. 1982: 244. Vinogradov 1999: 1176.
Cystosoma View in CoL – Bate, 1862: 311. WillemöesSuhm 1874a: 182. Gerstaecker 1886: 490.
Thaumops WillemöesSuhm, 1873: 206 View in CoL . – WillemöesSuhm 1874b: 634.
Thaumatops View in CoL – Martens, 1873: 189. Bovallius 1886: 3. Bovallius 1887b: 557. Bovallius 1889: 40. Stephensen 1918: 56. Schellenberg 1927: 620. Spandl 1927: 170. Pirlot 1929: 89.
Cysteosoma Bovallius 1886: 3 View in CoL .
Thaumonectes Senna, 1903: 93 View in CoL .
Physosoma Woltereck, 1904: 553 .
Type species
Cystisoma neptunus GuérinMéneville, 1842 , by monotypy. Type material could not be found at the MNHN, BMNH or ANSP and is considered lost. This is not an ideal situation since the true identity of C. neptunus is uncertain. However, there is no doubt that C. neptunus , as described and figured by GuérinMéneville (1842), belongs to the modern generic concept of Cystisoma View in CoL .
Synonyms
Cystosoma and Cysteosoma are variations in the spelling of Cystisoma .
WillemöesSuhm (1873) proposed the genus Thaumops for his new species T. pellucida , unaware that this was a synonym of Cystisoma . He subsequently (1875) realised his error. Martens (1873) corrected the spelling to Thaumatops .
Thaumonectes and Physosoma are names given to larval forms.
Sexual dimorphism
There are very few reliable morphological characters to distinguish the sexes of species of Cystisoma , and juveniles (<20 mm) are impossible to sex. The reproductive systems have been described by Brusca (1981b) and provide the only reliable means to distinguish the sexes. Unfortunately these are sometimes difficult to see in damaged specimens. Briefly, the male reproductive system consists of paired testes, suspended in pereonites 13, with paired sperm ducts extending posteriorly to pereonite 7, where they terminate in the gonopores, each elevated in a small papilla. Generally the male gonopores are readily visible in all but juvenile (<2030 mm) and damaged specimens. The female reproductive system is more complex. Ovaries are located in pereonites 34, and the oviducts terminate in gonopores on pereonite 5, which open on the medial side of small brood plates, which are pressed against the body. Mature specimens have a brood sac between the second gnathopods, which is covered by two pairs of brood plates arising posteriorly on pereonites 2 and 3 ( Fig. 3 View FIGURE 3 ). These brood plates are present as developing buds in immature specimens and provide a reliable character to distinguish females. Females as small as 30 mm can have the first two pairs of brood plates present as small buds. The presence of the gonopore is an additional character but is less obvious, especially in damaged specimens.
In addition to the above, in mature females, pereopod 7 is transformed into a prehensile appendage, with an expanded, glandfilled propodus and short hooked dactylus. Males possess a much narrower propodus without glands, and a longer straighter dactylus. As this character is only seen in mature females it is not very useful for distinguishing the sexes.
Various authors have also suggested other possible sexual differences such as the length of the first antennae; the head shape, the presence of glands in the antennae, pereopods and uropods, and the shape of the basis of pereopod 7. Sometimes smaller specimens have relatively longer first antennae and occasionally smaller males (4045 mm) have slightly longer antennae than females of the same size. Woltereck (1903) and Vinogradov et al. (1982) suggest that males may have more wedgeshaped heads, but this could not be verified from the material examined. In fact the type male of the new species described here has a rather rounded head. Adult females of C. pellucida have highly developed glands in the distal part of the propodus of pereopods 37, and apically on the first antennae and the exopods of the uropods. These glands are not evident in the females of other species and males of C. pellucida are unknown, so it is impossible to determine whether or not these glands are present. The only record of males of C. pellucida are by Pirlot (1938), Brusca (1967b) and Vinogradov et al. (1982), but they do not provide a description or illustrations, and these specimens were not available for examination. The basis of pereopod 7 is relatively broad in the males of two species, C. latipes and C. gershwinae sp. nov., for which the females are unknown. In all other species the basis of pereopod 7 is relatively slender, like the following articles, and there do not seem to be any sexual differences.
Remarks
Cystisoma is a readily recognisable genus, but distinguishing its species can be a frustrating process as they are surprisingly similar morphologically ( Brusca 1981b). Characters that might prove useful to distinguish species are as follows.
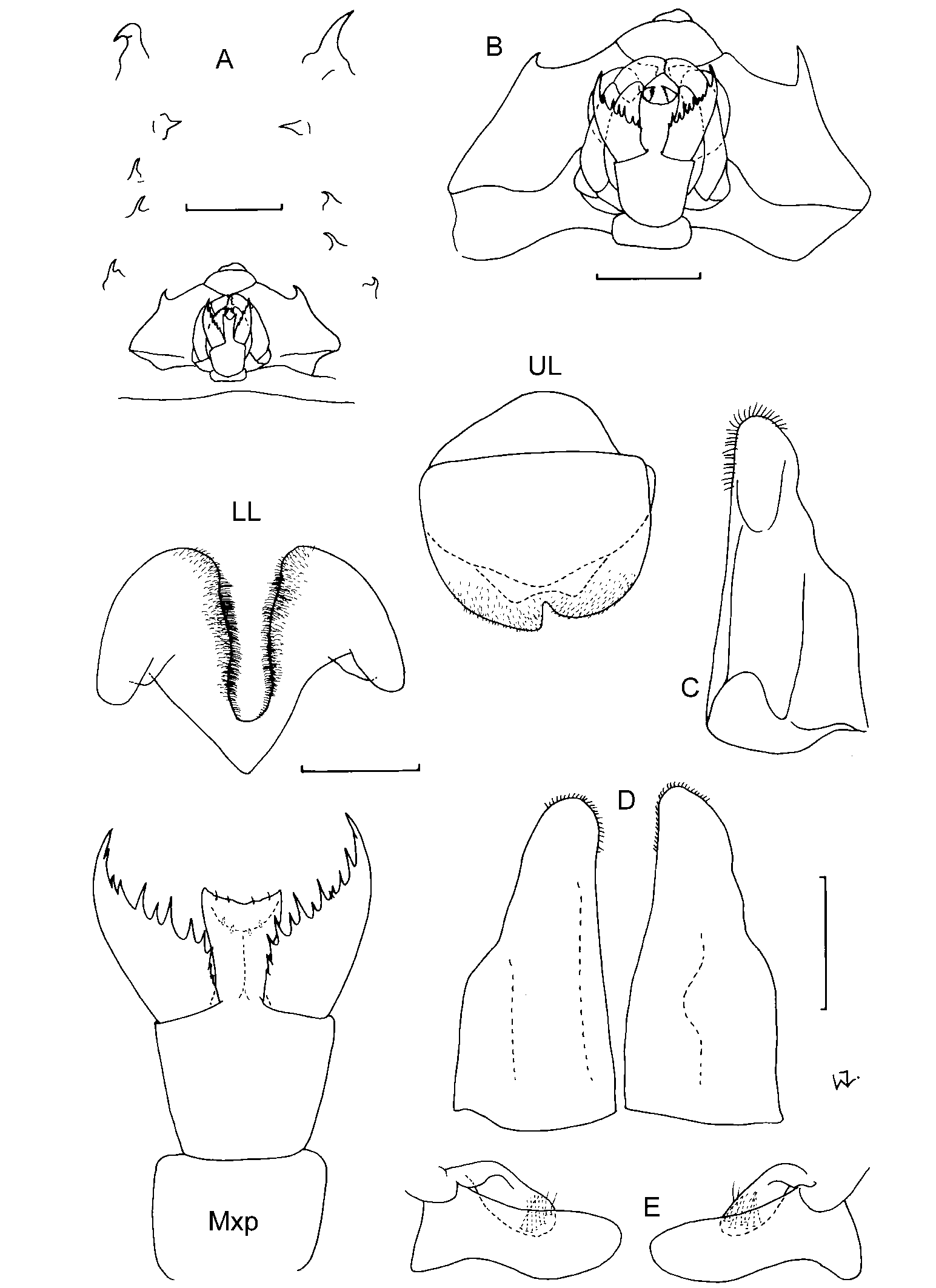
First antennae: The length of the first antennae seems to vary slightly with age, with juveniles tending to have relatively longer antennae. In adults the length of the first antennae seems to be a useful character. They are equal in length to about half of the head in C. magna ; 0.50.7x the head in C. longipes ; subequal to the head in C. fabricii and C. pellucida , much longer than the head, extending to the pleon, in C. latipes , and extending almost to pleonite 2 in C. gershwinae sp. nov. However, this is not always a reliable character as juvenile males (4045 mm) attributable to C. magna , C. longipes and C. fabricii occasionally have antennae considerably longer than the head, sometimes extending to pereonite 3. Apart from the long antennae, and smaller size, these specimens are like the adults and in the absence of other characters have been identified with them. Cystisoma latipes and C. gershwinae sp. nov. are also the only species to have antennae with a brush of aesthestascs on the inner surface. In all other species only a few scattered aesthestascs, or none at all, were found. The antennae of C. pellucida are swollen distally due to the presence of a gland, a distinguishing feature of this species.
Second antennae: The second antennae seem to be represented by small ventral spines posterior to the usually larger, anterior spines, on the ventral surface of the head. Stephensen (1918) who first classified the ventral spines concluded that the glandular spine is “undoubtedly a rudiment of ant. 2, and into which the antennal gland opens out”. A number of specimens were examined in which a gland is clearly attached to the glandular spine, confirming that this is probably the remnant of the second antennae. In C. latipes and C. gershwinae sp. nov. the glandular spine is similar in size to, or slightly larger than, the anterior spine. In all other species the anterior spine is clearly the largest ventral spine.
Head shape: When viewed dorsally, the head can be rounded with a convex anterior margin as in C. longipes and C. magna , or it may be more rectangular in shape, with a relatively straight, or almost concave anterior margin as, in all other species.
Eye shape: When viewed dorsally, the eye facets of C. latipes occupy two obliquely oval areas almost touching anteriorly, and widely separated posteriorly. In all other species the eye facets occupy most of the dorsal head surface, consisting of two almost oval areas barely separated along the middle of the head.
Marginal spines on head: The number of marginal spines increases slightly with an increase in size, and varies from eleven in C. gershwinae sp. nov. to 1418 in C. longipes . There is considerable overlap between species and this is not a useful character.
Ventral spines on head: The ventral spines consist of an anterior spine (usually the largest), followed by a glandular spine (most likely A2), and an arch of oral spines (in most species). The number of oral spines varies from 25. They are absent in C. fabricii and C. gershwinae sp. nov. Thus, the absence of oral spines could be used to distinguish these two species. It seems that oral spines are always present in the other species, with a likely increase in number with increase in size, although one unidentifiable juvenile, measuring only 17 mm, had 2 and 3 oral spines (‘Discovery’ specimen, unregistered BMNH).
Mouthparts: The mouthparts are remarkably similar except for the number of mandibular teeth. Most species have only one prominent, medial tooth on the mandible, rarely with a small adjacent one (e.g. C. fabricii , SAM A42204). Additional lateral teeth occur in C. latipes (one only), and C. longipes (13), and this character can be used to distinguish these two species.
Female brood plates: The brood plates of mature specimens of all species, except for C. latipes and C. gershwinae sp. nov., species for which the female is unknown, were examined and no differences were found. Because brood plates are not fully developed except for mature specimens, and are restricted to females, they are not a useful character anyway.
Pereopod length: There is very little variation in the relative lengths of the pereopods across the whole range of species regardless of sex or size.
Pereopod articles: Only minor variations were found in the relative lengths of pereopod articles. In C. fabricii and C. pellucida the propodus of pereopod 5 is clearly longer than the carpus; in all other species the propodus is subequal in length to the carpus. In C. gershwinae sp. nov. the carpus and propodus of pereopod 5 are subequal in length to the basis; in all other species the basis is clearly the longest article. In C. pellucida the propodus of pereopod 6 is slightly longer than the carpus; in all other species the carpus and propodus are subequal in length.
Pereopod 7 of females: In mature females this pereopod is modified, presumably for the transfer of eggs from the oviduct to the brood chamber. The propodus is swollen distally, forming a concave distal margin, and the dactylus is curved, presumably to hold the egg against the concave surface of the propodus. Although limited to females, Vinogradov et al. (1982) suggested that this might be a useful character, but the morphology of pereopod 7 changes considerably as females mature, and appear to be similar at the same stage in all species examined thus, making it an unreliable character.
Uropods: There is very little variation in the relative lengths of the peduncle and the exopods or endopods. In most species the exopods are slightly longer than the endopods but in C. pellucida the exopods are considerably longer and swollen distally because of the presence of a gland, making it a readily recognisable character. In uropod 1 the exopod length relative to the peduncle is about onethird in C. magna and onehalf in C. pellucida , with all other species inbetween (about 0.4x). In uropod 3 the exopod length relative to the peduncle is usually slightly more than half in most species, except for C. magna (slightly less than half) and C. pellucida (about twothirds).
There are twelve nominal species referable to Cystisoma . Of these, the type material of seven species has been confirmed as lost, but only two species, C. spinosus ( Fabricius, 1775) and C. neptunus GuérinMéneville, 1842 , are insufficiently described, making determination impossible. In addition, two names have been given to larval forms, Thaumonectes ducis aprutii Senna, 1903 and Physosoma Woltereck, 1904 .
Six species are recognised in this review including one described as new. All appear to inhabit relatively shallow waters (2001000 m) of the world’s oceans, tending towards the temperate and tropical regions. Occasionally specimens are caught in surface waters, or are found washed up onshore after storms. Very little is known about their biology. No gelatinous hosts have been recorded and their association with gelatinous plankton remains to be confirmed.
Because species of Cystisoma are very similar morphologically ( Brusca 1981b), detailed descriptions are not given, except for the species described here as new to science.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Cystisoma GuérinMéneville
| Zeidler, Wolfgang 2003 |
Physosoma
| Woltereck 1904: 553 |
Thaumonectes
| Senna 1903: 93 |
Cysteosoma
| Bovallius 1886: 3 |
Thaumops WillemöesSuhm, 1873 : 206
| Willemoes-Suhm 1874: 634 |
| Willemoes-Suhm 1873: 206 |
Thaumatops
| Pirlot 1929: 89 |
| Schellenberg 1927: 620 |
| Spandl 1927: 170 |
| Stephensen 1918: 56 |
| Bovallius 1889: 40 |
| Bovallius 1887: 557 |
| Bovallius 1886: 3 |
| Martens 1873: 189 |
Cystosoma
| Gerstaecker 1886: 490 |
| Willemoes-Suhm 1874: 182 |
| Bate 1862: 311 |
Cystisoma GuérinMéneville, 1842 : 215
| Vinogradov 1999: 1176 |
| Vinogradov 1982: 244 |
| Bowman 1973: 26 |
| Pirlot 1939: 33 |
| Pirlot 1938: 364 |
| Barnard 1932: 268 |
| Barnard 1916: 286 |
| Stebbing 1888: 1318 |
| Suhm 1875: 24 |
| Dana 1853: 981 |
| Dana 1852: 315 |
| Guerin-Meneville 1842: 215 |