Cornutipo chillagoensis, Semeraro & Constant, 2023
|
publication ID |
https://doi.org/ 10.5281/zenodo.10981827 |
|
publication LSID |
lsid:zoobank.org:pub:A992D038-1989-4284-BB0E-91C77D745317 |
|
DOI |
https://doi.org/10.5281/zenodo.11045970 |
|
persistent identifier |
https://treatment.plazi.org/id/D976CBD6-AD9F-4A34-B2EC-F4B1329C7BE4 |
|
taxon LSID |
lsid:zoobank.org:act:D976CBD6-AD9F-4A34-B2EC-F4B1329C7BE4 |
|
treatment provided by |
Felipe |
|
scientific name |
Cornutipo chillagoensis |
| status |
sp. nov. |
Cornutipo chillagoensis sp. nov.
urn: sp.nov.: urn:lsid:zoobank.org:act:D976CBD6-AD9F-4A34-B2EC-F4B1329C7BE4
( Figs 1-6 View Fig View Fig View Fig View Fig View Fig View Fig , 8 View Fig )
ETYMOLOGY.
This species is named after the town, Chillagoe, North Queensland, Australia, from which the specimens were collected, in bushland near the Chillagoe Observatory and Eco Lodge.
TYPE MATERIAL.
HOLOTYPE
AUSTRALIA • ♂ ( Figs 1 D View Fig , 5 View Fig ); Queensland, Chillagoe; 17°08’55”S, 144°31’43”E; 7–11 May 2022; alt. 400–500m; on Acacia auriculiformis ; J. Constant & L. Semeraro leg.; specimen tended by ants; “Australia Qld. Chillagoe, 17°08’55”S, 144°31’43”E, 7–11 May 2022, alt. 400– 500m, leg. J. Constant & L. Semeraro, Leopold III Funds EXpedition”, “collected on Acacia auriculiformis ; specimen tended by ants”; QM. GoogleMaps
GoogleMapsPARATYPES
AUSTRALIA • 2♂♂, 5♀♀, 1 seX undet. (abdomen missing); collection details as per holotype; QM • 2♂♂, 5♀♀; collection details as per holotype; RBINS.
DIAGNOSIS.
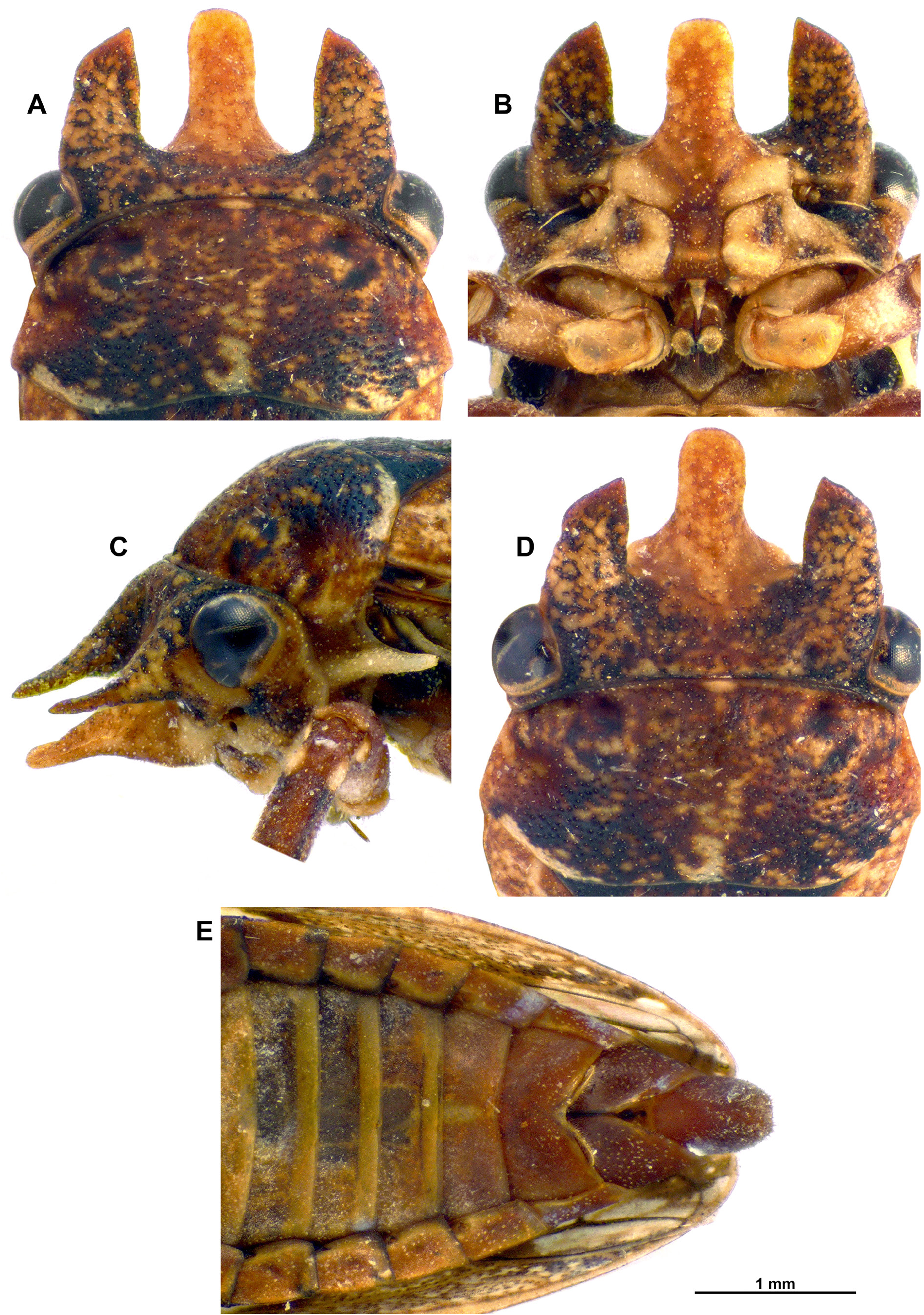
Generally mottled, pale to dark brown and stramineous with strikingly contrasted white to cream coloured oblique fascia basally and preapically on tegmen ( Figs 1 View Fig , 3 View Fig ). Head with three horns, all about equal or subequal in length when viewed dorsally, fronto-clypeal horn, widest at base, more or less parallel sided throughout, truncate at apeX ( Fig. 2A View Fig , 4A View Fig ). Aedeagus with two apical processes and a pair of preapical processes ( Fig. 5 View Fig H-L).
DESCRIPTION.
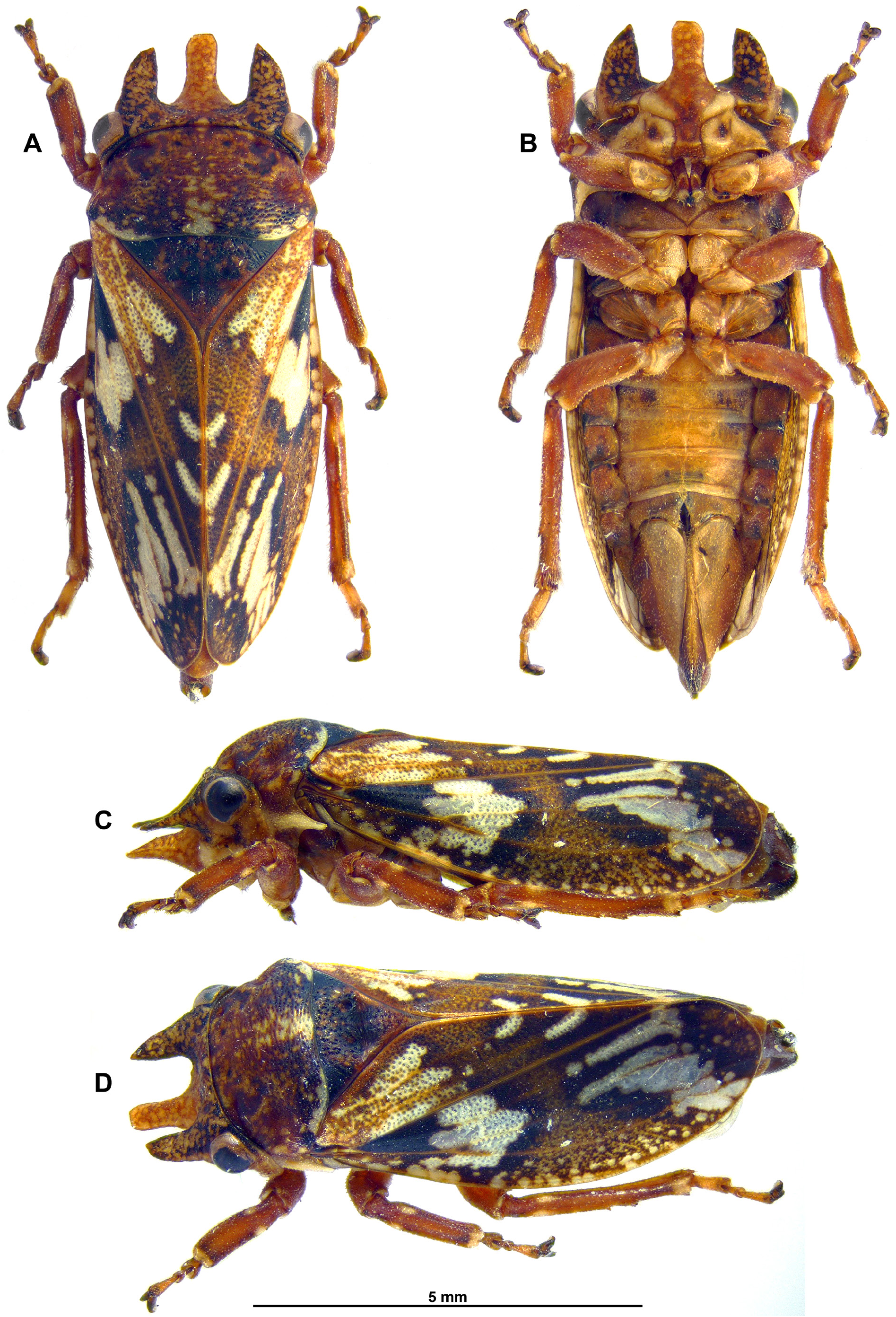
Measurement and ratios: Body length, ♂ holotype, 7.5mm; 4 ♂♂, 7.4–7.8mm; 10 ♀♀, 7.9–8.7mm. Head equal to sub-equal width of pronotum. Pronotal length about 2/5 to 3/5 its width. Tegmen length 2.4–2.8 times medial width. Length of metatibiae around 1.5X length of mesotibiae; mesotibiae 1.5X length of mesotarsi. Female pygofer and ovipositor ventral length, 2.3–2.7mm (n=8).
Head: ( Figs 2 View Fig A-D, 4A-C) Mottled with stramineous background and orange brown to dark brown reticulate patches; face mottled dark brown to black patches, lora and posterior lateral frontoclypeus stramineous to cream coloured, with dark brown patch laterally. Tiny scale-like setae on face, particularly around gena and lora; three horns—medial frontoclypeal horn, widest at base, lateral margins more or less parallel sided throughout (after basal ¼), width at mid-length as wide as at apeX, apically roundly truncate, lateral horns on verteX equal to sub-equal in length compared to medial frontoclypeal projection when viewed dorsally, inner lateral margins of each, straight to very weakly conveX, outer lateral margins slightly conveX, strongly conveX apically, tapering to a point towards inner margin; ocelli medial on face, positioned in slight concavity between lateral horns on verteX; antennal bases closer to posterior lateral margin of lora than to posterior corner of eyes. Rostrum reaching posterior margin of procoXae or up to anterior margin of mesocoXae; uniformly brown.
Thorax: ( Figs 1 View Fig A-C, E, 2A-D, 3, 4A-C) Pronotum pitted and coloration mottled as per head; slightly wider posteriorly than anteriorly, with coronal calli depressed and a further pair of depressions along lateral mid-length of pronotum; lateral pronotal margin long, well separating the head from the base of the tegmen; posterior margin slightly concave medially. Pro-epimeron distinctly eXtended posteriorly into a narrow tapering spine. Mesonotum and scutellum pitted and mottled dark brown, darker than pronotum, anterior lateral angles with dark brown to black triangles, apeX orange to brown, stramineous apically.
Tegmina: ( Figs 1A, C, E View Fig , 3A View Fig , C-D) Mottled with dark brown to light brown patches, opaque white to cream fascia anteriorly eXtending obliquely from base of clavus across Cu, M and R veins almost to costal margin, and semi-translucent oblique fascia posteriorly at around ¾ length of tegmen from apeX of claval suture, crossing pre-apical cells; distal portion of anal veins cream coloured, appearing like a v-shaped pattern from dorsal aspect; punctate particularly visible around base of clavus. Tegminal veins generally light brown.
Hind wings: ( Figs 1 D View Fig , 4 E View Fig ) Mostly infuscate and translucent, fuscous along vein Cu2 and anal vein 1; veins brown to dark brown; small wing coupling process along anterior margin dark brown to black.
Legs: ( Figs 1 View Fig A-C, E, 3A-D) Mostly brown; a few very small, almost scale-like setae on legs; tibiae on all legs dorsally flattened; protibiae relatively broad, width around 0.3–0.4X length; white to cream coloured lateral spots at around basal 1/3 of each tibia on dorsal aspect, white to cream coloured ring at apeX of tibiae on all legs; 2 spurs on hind tibia, most distal spur largest; tarsal claws relatively large, slightly less than 0.5X length of third tarsomere.
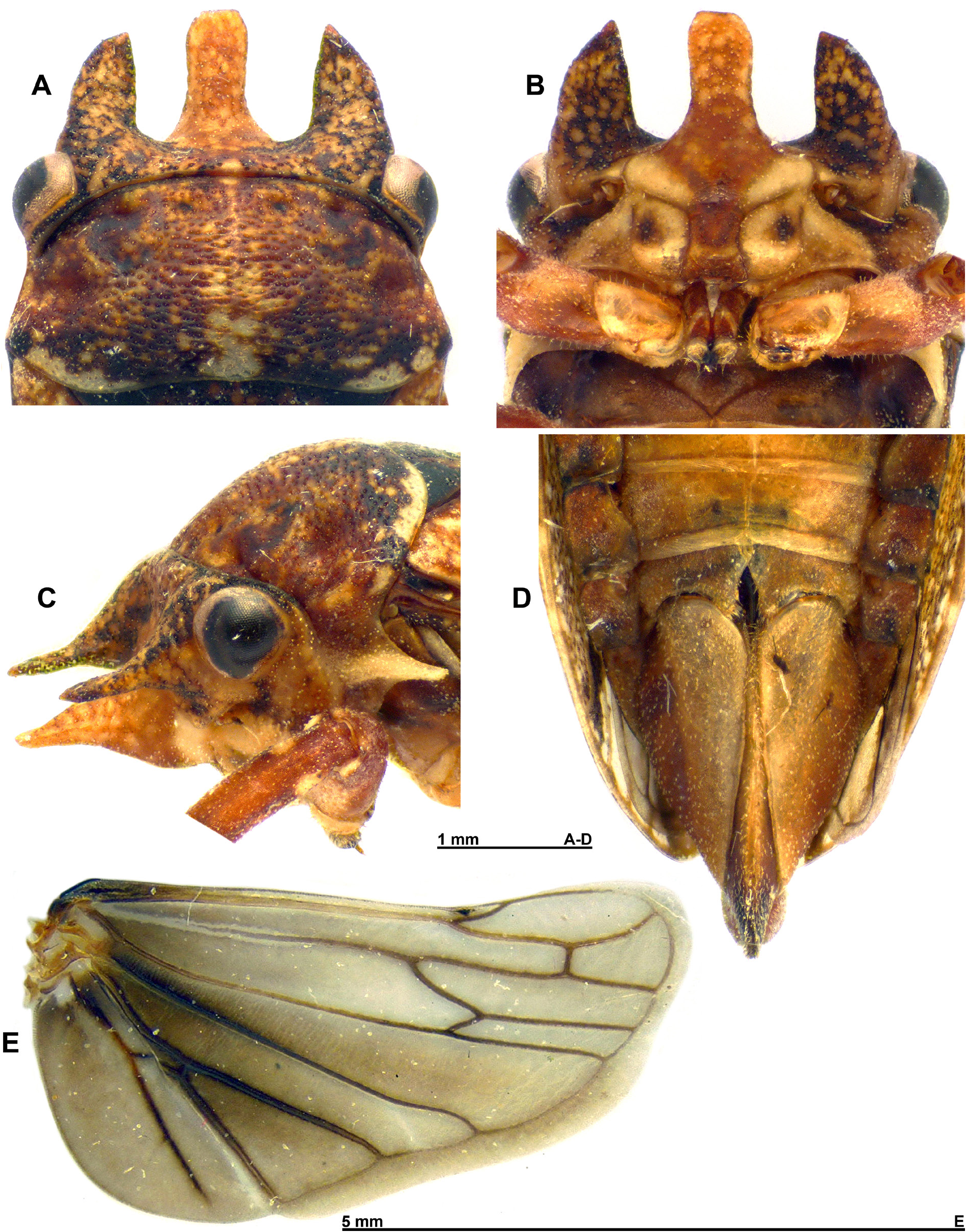
Abdomen: ( Figs 1 B View Fig , 2 E View Fig , 3 B View Fig , 4 D View Fig ) Mostly brown; tergites mostly dark brown, posteriorly paler orange brown; ventrally stramineous, may be orange to pale brown towards posterior sternites. Terminalia ♂: ( Figs 2 E View Fig , 5 View Fig ) Pregenital sternite generally quadrate, lateral margins parallelsided, posterior margin, broadly and distinctly v-shaped medially. Pygofer dark brown, slightly paler anteriorly, higher than wide; anterior margin broadly concave; posterior margin roughly quadrate, slightly concave along ventral half; dark brown sclerotized section along anterior ventral margin; posteroventral margin pre-apically strongly notched with a distinct concavity, ventroapically rounded; anal tube large, height almost half that of pygofer and triangular-shaped in lateral view; dorsoposterior margin oblique; small short, fine setae all over anal tube and on pygofer. Aedeagus, laterally flattened with apeX directed ventrad; thimble-shaped sac at base of aedeagus; aedeagal anterior margin with triangular-shaped hump medially and smaller hump towards base; posterior margin basally constricted, evenly conveX along the rest of its length; two apical processes anterior to gonopore; in lateral view, anterior-most process long, straight directed anteroventrad; a pair of small fine lateral pre-apical processes, at base of anterior-most apical process, about 1/3 of its length; posterior process, curved, directed laterodorsad. Subgenital plates short, not quite reaching apical point of pygofer, apically strongly rounded; dorsoapically produced into a strongly sclerotized tooth. Paramere apeX, reaching almost half of subgenital plate length, apically tapered, knife-shaped tapering with blunt apices, indentation along inner margin.
Terminalia ♀: ( Fig. 4 D View Fig ) Tegmina not reaching apeX of abdomen in females; anal tube, pygofer and ovipositor slightly eXposed distally. Pregenital sternite posterior margin, medially divided by deep, narrow v-shaped notch, almost splitting the sternite in two; posterior margin produced medially on each side of notch, appearing M-shaped, a deep concavity on each side of posterior margin, tufts of setae on posterior medial projections. Ovipositor distinctly eXceeding apical point of pygofer posterior margin. Anal tube large (as in males) reaching around half height of pygofer.
COMMON NAME.
The Triceratops leafhopper. Bearing three horns on the face, this configuration is reminiscent of the three horned Triceratops dinosaur.
BIOLOGY.
The specimens of C. chillagoensis sp. nov. were collected in dry open woodland, with mostly Eucalypts ( Fig. 6 D View Fig ). This species was found being attended by ants ( Fig 6 View Fig B-C) during the day (late afternoon) on the small, presumably young Acacia auriculiformis shrubs (pers. com J. Wainer, Nov. 2022). Two different species of ants of the subfamily Dolichoderinae ( Formicidae ) were collected but only one species of ant was found attending the adult leafhopper at any one time. One of the ant species was identified as Papyrius nitidus (Mayr, 1862) (pers. com J. Wainer, Nov. 2022 – Figs 6 C View Fig , 7 View Fig A-B), which is previously recorded tending scale insects (Coccoidea) and butterfly caterpillars (Lepidoptera) ( ANTWIKI, 2022). The second ant species was Iridomyrmex sp. , possibly I. sanguineus Forel, 1910 (pers. com J. Wainer, Nov. 2022 – Figs 6A View Fig , 7 View Fig C-D), a common meat ant ( ANTWIKI, 2022) with some species in this genus also known to attend myrmecophilous caterpillars, aphids and scale insects ( ANTWIKI, 2022). No leafhopper nymphs were observed.
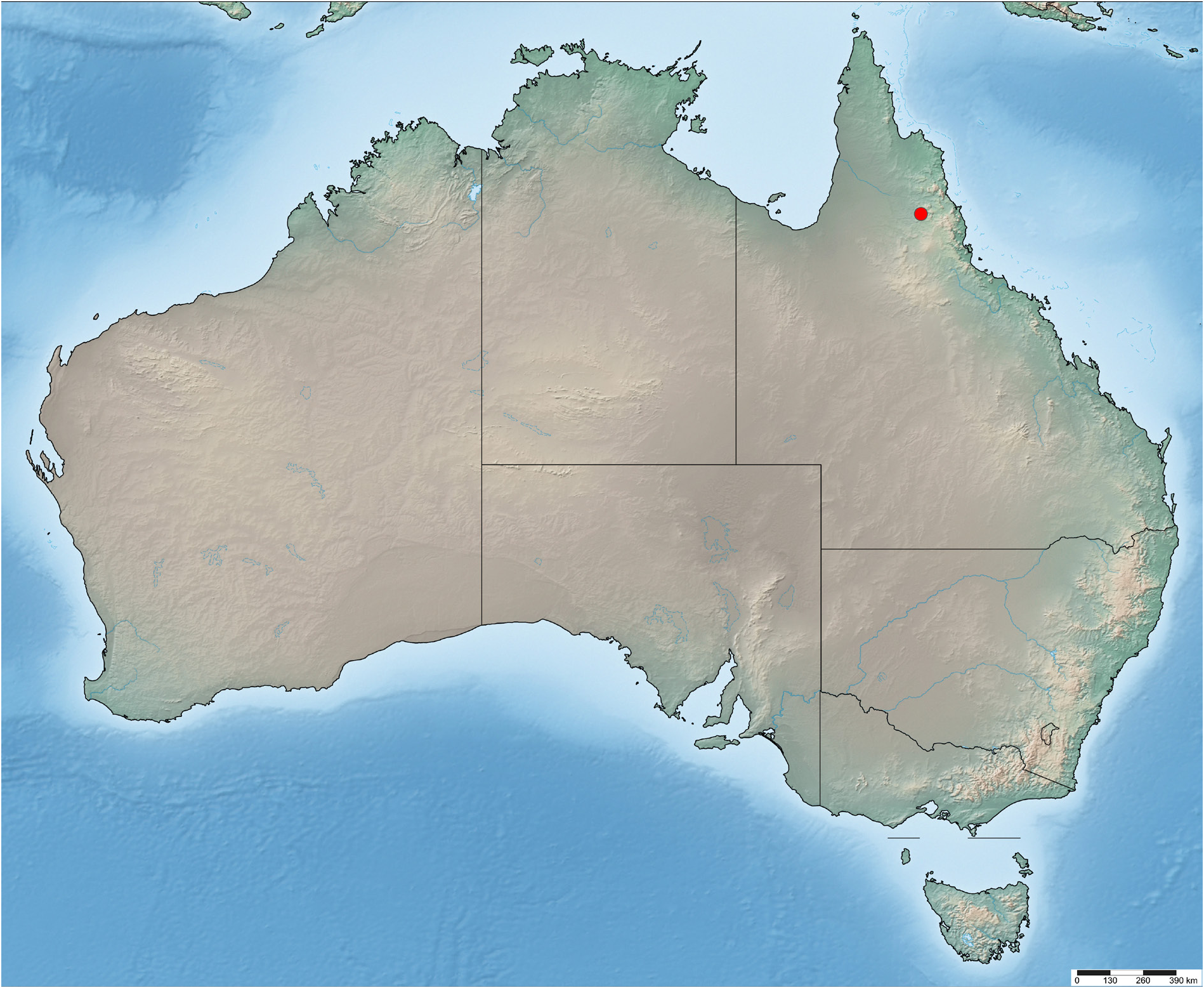
DISTRIBUTION.
Australia: Queensland, Chillagoe ( Fig. 8 View Fig ).
HOST. Acacia auriculiformis A. Cunn. eX. Benth. ( Mimosaceae ) ( Fig. 6 View Fig D-E).
DIFFERENTIAL DIAGNOSIS.
Both C. chillagoensis sp. nov. and C. tricornis differ from the other two Cornutipo species in having three horns on the head, while C. bakeri ( Evans, 1969) has two smaller acutely pointed processes laterally on the verteX with a slightly upturned posterior margin of the frontoclypeus and C. scalpellum ( Evans, 1934) has a single spatulate process on the posterior margin of the frontoclypeus but no lateral horns on the verteX. The shape of the aedeagi also differ appearing pick-shaped at apeX in both C. bakeri and C. scalpellum with no apical processes while C. tricornis and C. chillagoensis sp. nov. have two apical and a pair of pre-apical processes. The males of C. bakeri and C. scalpellum are smaller in size (5–5.6mm) compared with C. tricornis and C. chillagoensis sp. nov. (6–7.8mm).
Cornutipo chillagoensis sp. nov. is most similar in appearance to C. tricornis but in the latter, the medial horn, arising from the frontoclypeus, is tapered towards the apex and the two lateral horns of the verteX are distinctly shorter than the medial horn when viewed dorsally, while in C. chillagoensis sp. nov. the medial horn is more or less parallel sided and truncate at the apeX and the lateral horns appear equal to subequal in length to the medial horn in dorsal view. In lateral view, the two lateral horns are curved ventrad in C. tricornis , but directed anteriad in C. chillagoensis sp. nov. The colouration of C. tricornis differs from C. chillagoensis sp. nov. in having less pale brown or orange brown and white to cream coloured markings on the tegmina which are mostly mottled dark brown in comparison to C. chillagoensis sp. nov. which have distinct white fasciae on the tegmen. Also, based on the description ( EVANS, 1934), C. tricornis is considerably smaller in body size (6mm) than C. chillagoensis sp. nov., which ranges in size from 7.4–8.7mm (average ♂ = 7.6mm, ♀ = 8.3mm) and the width of the head is narrower and measured at 2.2mm in C. tricornis while it is 2.6–3mm in C. chillagoensis sp. nov.
The aedeagus of C. chillagoensis sp. nov., appears very similar in shape to C. tricornis with the apical processes being almost identical, however, the posterior-most process is more curved and appears slightly shorter in C. chillagoensis sp. nov. which also have a smaller but distinct pair of pre-apical processes at the base of the anterior-most apical process. While the preapical processes are neither described nor figured in EVANS (1934) for C. tricornis , at least one is visible in the genitalia of the holotype specimen ( Fig. 9 D View Fig ) and based on the paratype specimen appear to be relatively thick and long (longer than 1/3 length of anterior apical process), unlike C. chillagoensis sp. nov., in which the preapical processes are thinner and less than 1/3 length of anteroapical process; the subgenital plates bear an apical dorsal tooth which is strongly sclerotized and appears larger in C. chillagoensis sp. nov. compared with C. tricornis ; the parameres also differ in this species with C. chillagoensis sp. nov. having a distinct indentation along ¾ of the length of the inner margin.
COMMENTS.
In his original description of the genera Cornutipoides and Cornutipo, EVANS (1934) describes the metatibia as bearing one spur and in the case of Cornutipoides one spur and a few small spines. However, in C. chillagoensis sp. nov., the metatibia bears 1–2 spurs with one smaller than the other. No distinct seXual dimorphism is found in this species apart from the size, with females slightly larger than males. The function of the three horns on the head is unknown and the horns are the same shape and relative size between males and females.
| QM |
Queensland Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Auchenorrhyncha |
|
SuperFamily |
Membracoidea |
|
Family |
|
|
SubFamily |
Eurymelinae |
|
Tribe |
Ipoini |
|
Genus |